Preparation method and application method of chlamydia pneumoniae recombinant antigen
A technology of Chlamydia pneumoniae and recombinant antigen, which is applied in the field of medical drugs, can solve the problems of infection, slow detection speed and low accuracy of preparation personnel, and achieves the effects of low cost, simple operation and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
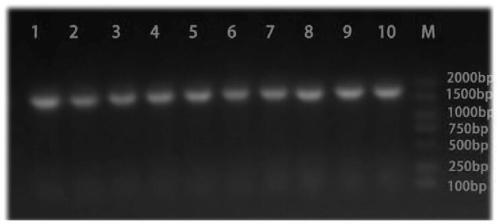
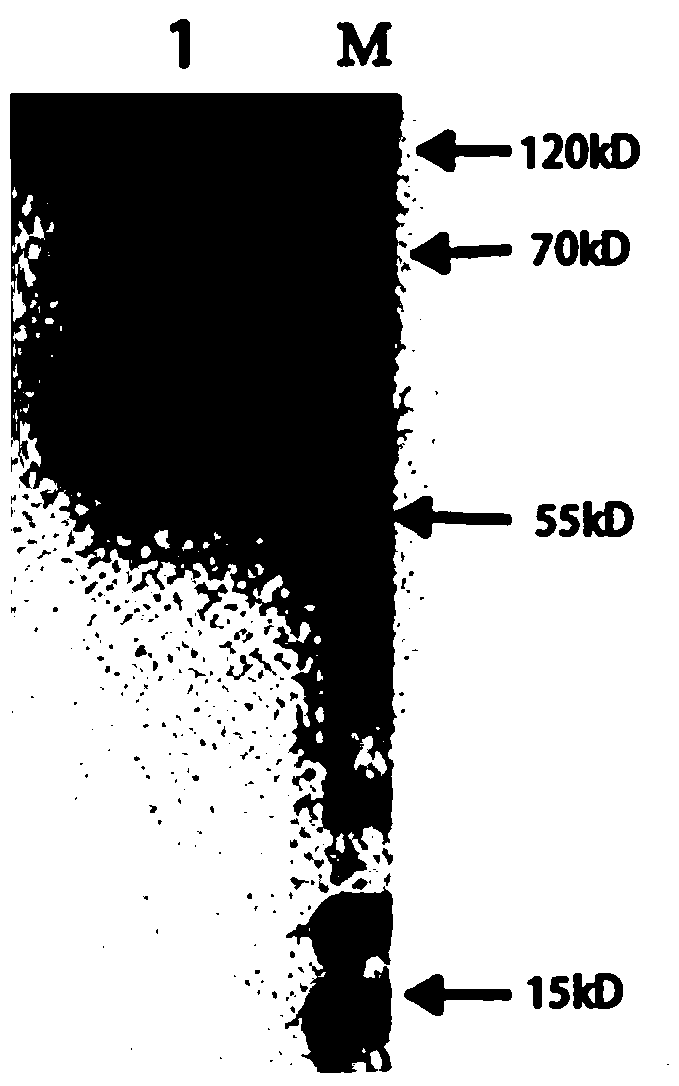
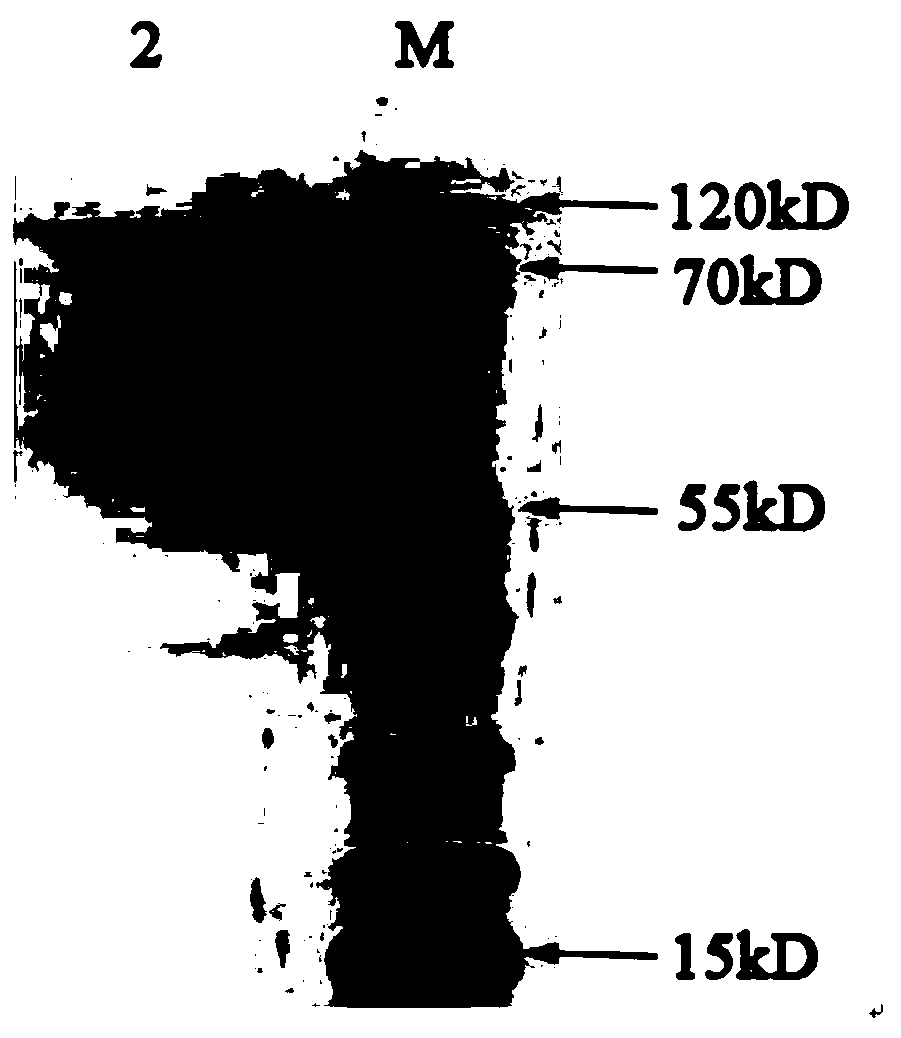
[0035] see figure 1 As shown, a preparation method of Chlamydia pneumoniae recombinant antigen, the specific steps are as follows: (1) Selection of Chlamydia pneumoniae recombinant antigen sequence: comprehensively compare the antigen targets reported in the existing literature of CP, and combine bioinformatics analysis to finally determine the incA , the most immunogenic amino acid segments of incB and opmA are: amino acid 220-amino acid 490 of incA, amino acid 98-amino acid 175 of incB gene, amino acid 25-amino acid 175 of ompA gene Amino acids at position 192 are connected in series through the connecting peptide GSGSGS. The series sequence from N-terminal to C-terminal is incA fragment-GSGSGS-incB fragment-GSGSGS-ompA fragment. The final nucleic acid sequence is obtained by chemical synthesis.
[0036] See SEQ ID NO.1 for the nucleotide sequence of the CP recombinant antigen, and SEQ ID NO.2 for the amino acid sequence. After chemically synthesizing the target gene fragmen...
Embodiment 2
[0047] see figure 2 , 3 Shown, a kind of application method of Chlamydia pneumoniae recombinant antigen, its specific steps are as follows:
[0048] (1) Dilute the CP protein to 2 μg / mL with coating buffer (0.1 mol / L carbonate buffer at pH 9.6), add it to the well of the microtiter plate, 100 μL / well, incubate at 37°C for 2 hours; shake off After coating, pat dry, wash with PBST 3 times, 3min / time; add 200μL of blocking solution (containing 2% bovine serum albumin phosphate buffer at final concentration) to each well, incubate at 37°C for 2h; shake off the bag After soaked, pat dry, and wash 3 times with PBST, 3min each time; at this time, it can be stored in vacuum packaging in aluminum foil bags;
[0049] (2) Dilute the sample to be tested 1:100 with the sample diluent and mix well;
[0050] (3) Add 100 μL of diluted sample to each well, and add 100 μL of CP-IgM calibrator solution to the reserved calibrator wells. After sealing the wells of the microplate plate, incubat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com