Hydrogen sulfide recognition and detection fluorescent probe, preparation method and applications thereof
A fluorescent probe and hydrogen sulfide technology, applied in the field of chemical analysis, can solve the problems of inability to detect hydrogen sulfide, and achieve the effect of fast response and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
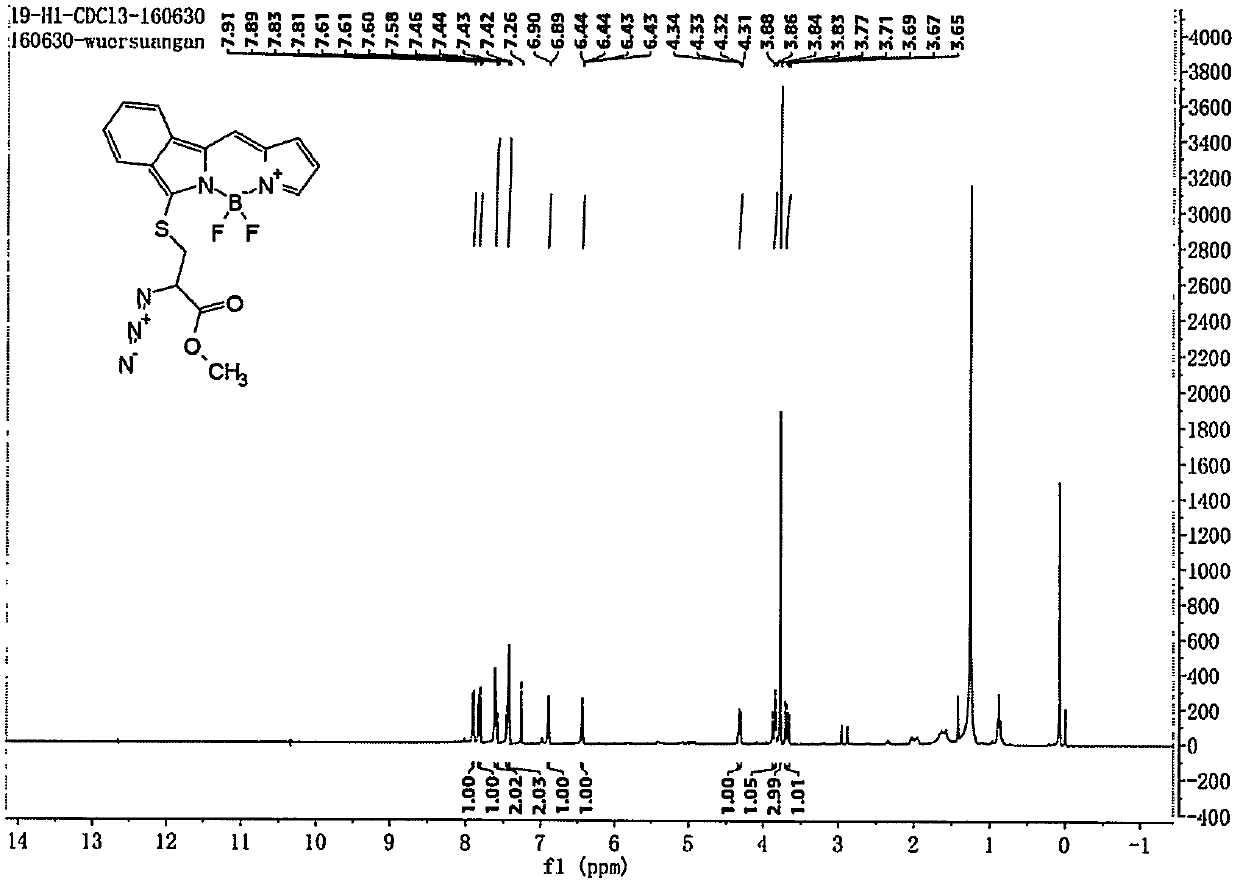
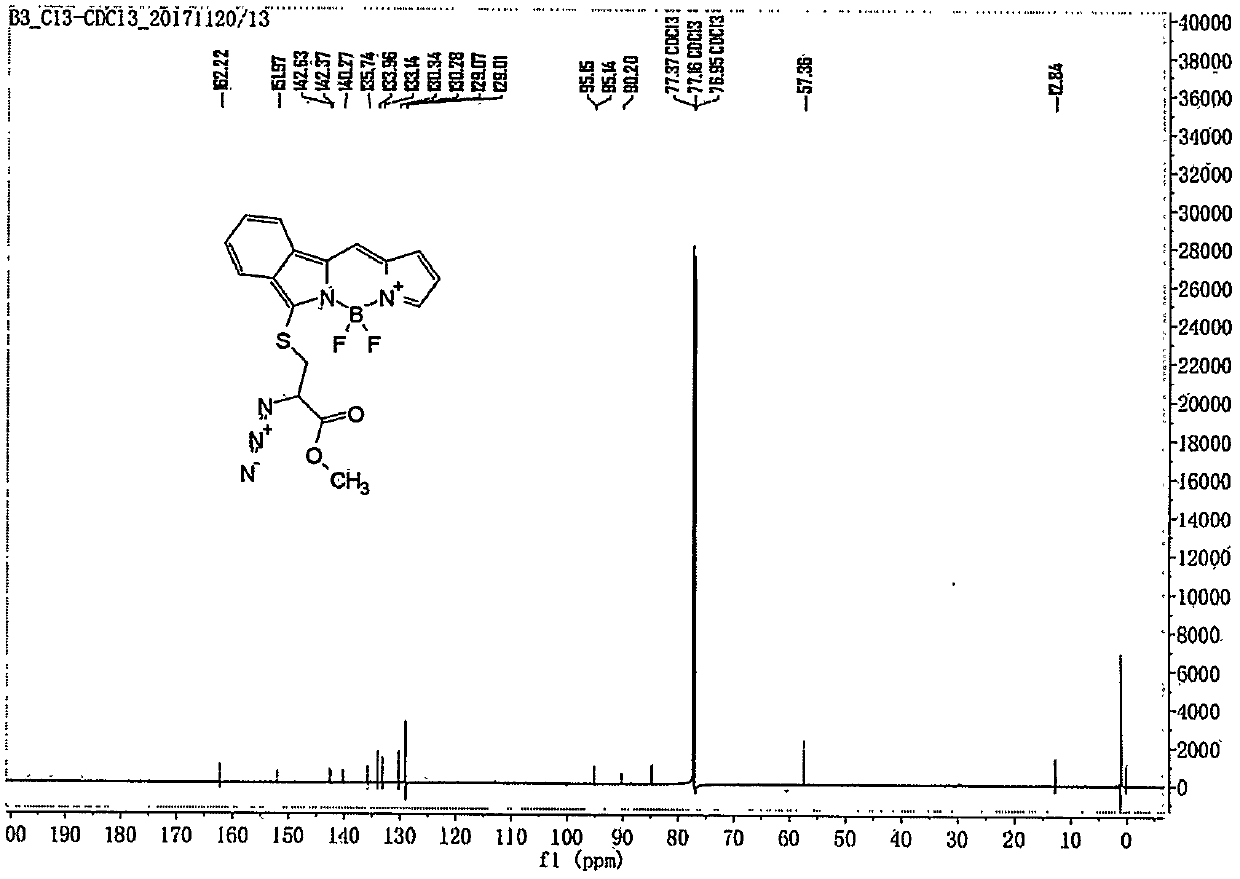
Embodiment 1
[0031] Preparation of compounds of general formula (1):
[0032]
[0033] Follow the steps below:
[0034] 1) Preparation of compound L2-1:
[0035]
[0036] Dissolve DMF (2.1mL, 27.3mmol) in 5mL of dichloromethane, and slowly add phosphorus oxychloride (2.3mL, 25mmol) in dichloromethane solution dropwise at 0°C, compound A (1.65g, 12.4mmol) Dichloromethane solution was added dropwise to the above solution, and refluxed at 45°C for 6h. The reaction was cooled to room temperature, 5M NaOH solution was added under an ice-water bath to adjust the pH to 8, the layers were separated, the organic layer was washed 3 times with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and passed through a neutral alumina column (PE:EA= 8:1), to obtain 1.35 g of bright yellow solid L2-1 with a yield of 53%. LC-MS (ESI) m / z: 207.1 [M] + ;
[0037] 2) Preparation of compound L2-2:
[0038]
[0039] Compound L2-1 (898mg, 5mmol) was dissolved in NaOH in eth...
Embodiment 2
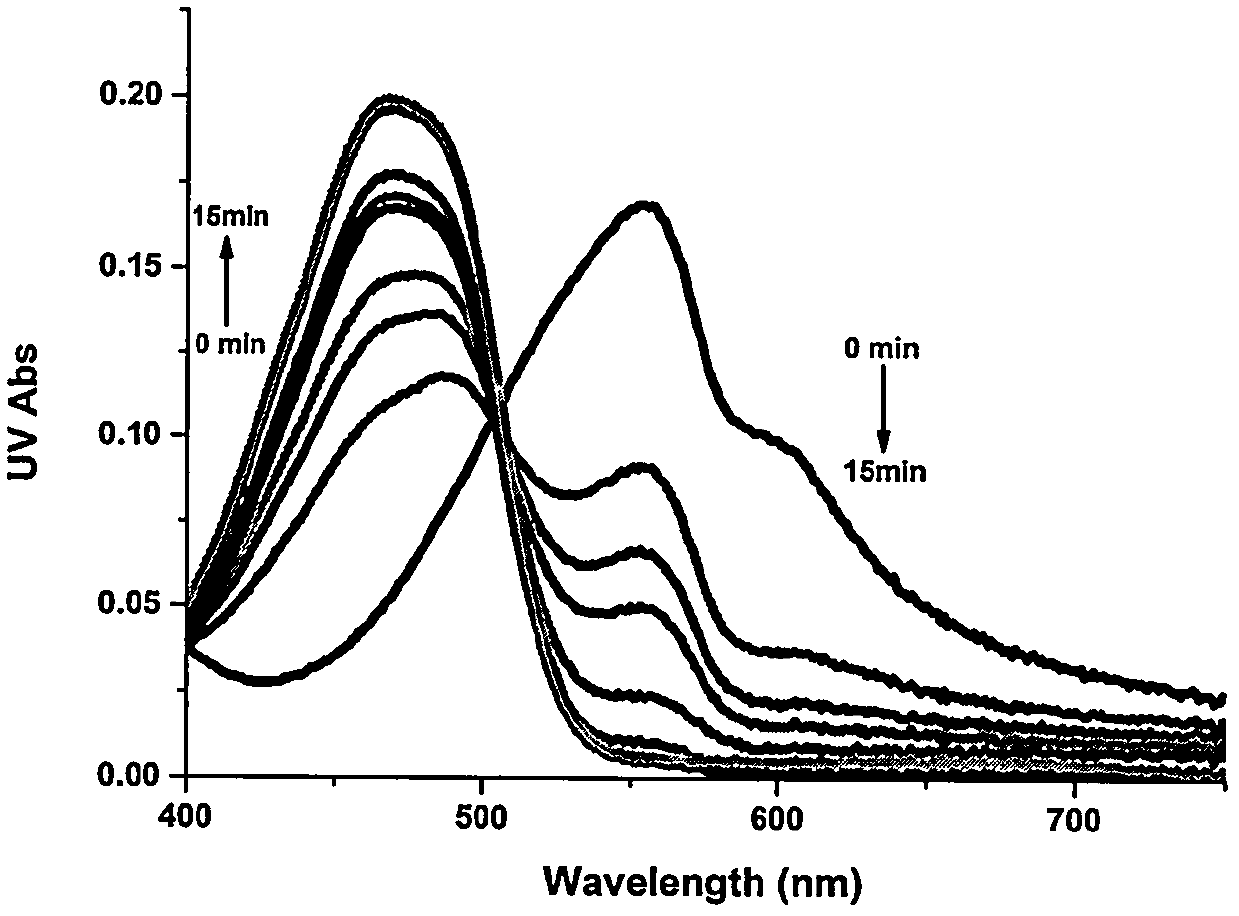
[0056] Example 2 Changes in the ultraviolet absorption spectrum of the probe (1) in response to hydrogen sulfide
[0057] Add 3 mL of PBS (10 mM, pH 7.4) to the quartz cuvette for zero baseline adjustment, then add 10 μL of L2-B acetonitrile stock solution to make the final concentration 10 μM, and then add 5 times the equivalent of NaSH solution to test the difference Changes in UV absorption of time probe L2-B reacting with hydrogen sulfide. Depend on image 3 It can be seen that with the prolongation of time, the absorption peak of the probe at 555nm gradually decreases, while the ultraviolet absorption at 470nm gradually increases, reaching the peak at 15min.
Embodiment 3
[0058] Example 3 Fluorescence Spectrum Change of Probe (1) Responding to Hydrogen Sulfide
[0059] Add 3mL of PBS (10mM, pH7.4) to the quartz cuvette, then add 10μL of L2-B acetonitrile stock solution to make the final concentration of 10μM, then add 2.5 times the equivalent of NaSH solution, detect probe L2-B and Fluorescence-time variation of the hydrogen sulfide response. Depend on Figure 4 It can be seen that as the reaction time prolongs, the fluorescence of the probe at 576nm is gradually quenched, and a new peak appears at 542nm, and the reaction reaches equilibrium in 15min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com