Nitrogen-containing fused tricyclic derivative and application thereof
A compound, hydrate technology, applied in Parkinson's disease. , The field of nitrogen-containing fused tricyclic derivatives and pharmaceutical compositions containing these compounds can solve problems such as adenosine A distribution limitation, and achieve the effects of good brain/plasma ratio, good bioavailability, and good safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
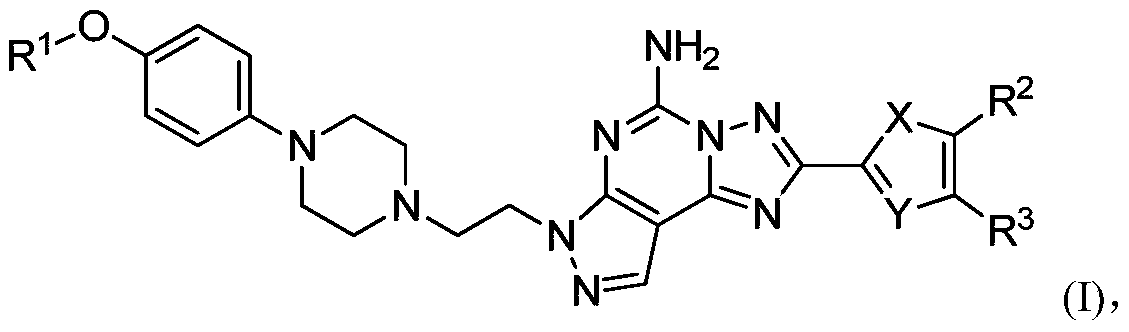
[0289] Example 1 2-(furan-2-yl)-7-(2-(4-(4-(oxetan-3-yloxy)phenyl)piperazin-1-yl)ethyl)- Synthesis of 7H-pyrazol[4,3-e][1,2,4]triazol[1,5-c]pyrimidin-5-amine
[0290]
[0291] Step 1) Synthesis of 2-amino-4,6-dichloropyrimidine-5-carbaldehyde
[0292]
[0293] Phosphorus oxychloride (58.7mL, 630mmol) was added to a 500mL single-necked round bottom flask, and N,N-dimethylformamide (12.1mL, 157mmol) was added dropwise in a low temperature bath at 5°C, and then transferred to 25°C , added 2-amino-4,6-dihydroxypyrimidine (10 g, 78.7 mmol) in batches, and raised the temperature to 100° C. to react for 5 hours. Stop the reaction, remove most of the phosphorus oxychloride by rotary evaporation under reduced pressure, add water (900mL), stir at 25°C for 32 hours, filter, add the obtained solid into ethyl acetate (50mL), stir for 30 minutes, Filtration and drying of the filter cake afforded the title compound as a yellow solid (12.2 g, 80.8%).
[0294] MS(ESI,pos.ion)m / z:192...
Embodiment 2
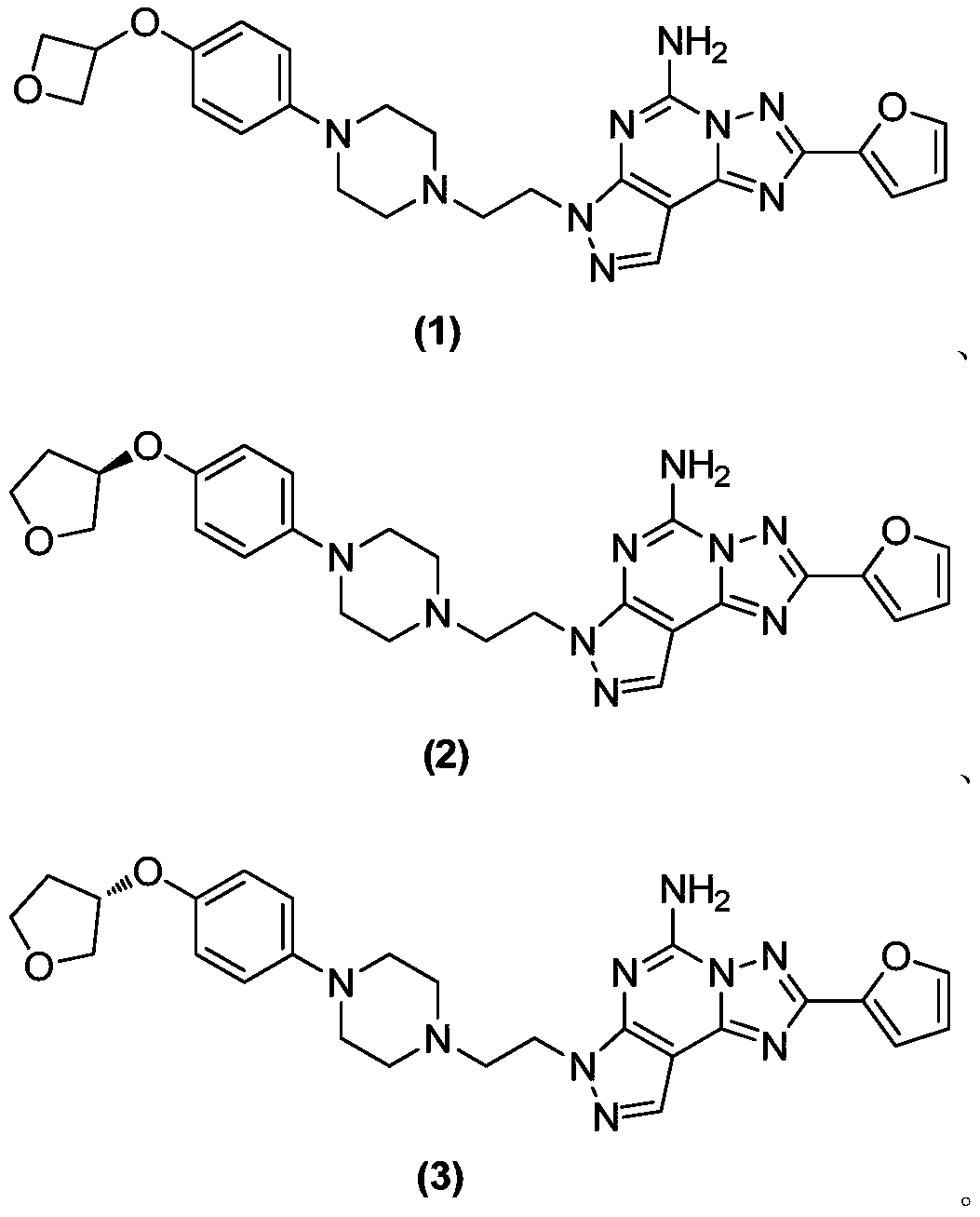
[0315] Example 2 (R)-2-(furan-2-yl)-7-(2-(4-(4-((tetrahydrofuran-3-yl)oxy)phenyl)piperazin-1-yl)ethyl Synthesis of -7H-pyrazol[4,3-e][1,2,4]triazol[1,5-c]pyrimidin-5-amine
[0316]
[0317]The title compound of this step was prepared by referring to the method described in step 6 of Example 1, that is, 2-(5-amino-2-(furan-2-yl)-7H-pyrazol[4,3-e][1,2 ,4] Triazol[1,5-c]pyrimidin-7-yl)ethyl 4-methylbenzenesulfonate (0.708g, 1.61mmol), (R)-1-(4-((tetrahydrofuran-3 -yl)oxy)phenyl)piperazine (0.68g, 2.74mmol) and N,N-diisopropylethylamine (0.8mL, 4.85mmol) in N,N-dimethylformamide (20mL) The reaction was prepared, and the crude product was separated and purified by silica gel column chromatography (dichloromethane / methanol (v / v)=20 / 1) to obtain the title compound as a white solid (378 mg, 45.5%).
[0318] MS(ESI,pos.ion)m / z:516.3[M+H] + ;
[0319] 1 H NMR (400MHz, DMSO-d 6 )δ(ppm)8.16(s,1H),8.05(s,2H),7.93(s,1H),7.23(d,J=3.1Hz,1H),6.84(d,J=9.1Hz,2H), 6.77(d, J=9.1Hz, 2H), ...
Embodiment 3
[0320] Example 3 (S)-2-(furan-2-yl)-7-(2-(4-(4-((tetrahydrofuran-3-yl)oxy)phenyl)piperazin-1-yl)ethyl Synthesis of -7H-pyrazol[4,3-e][1,2,4]triazol[1,5-c]pyrimidin-5-amine
[0321]
[0322] The title compound of this step was prepared by referring to the method described in step 6 of Example 1, that is, 2-(5-amino-2-(furan-2-yl)-7H-pyrazol[4,3-e][1,2 ,4] Triazol[1,5-c]pyrimidin-7-yl)ethyl 4-methylbenzenesulfonate (0.5g, 1.14mmol), (S)-1-(4-((tetrahydrofuran-3 -yl)oxy)phenyl)piperazine (0.424g, 1.71mmol) and N,N-diisopropylethylamine (0.38mL, 2.3mmol) in N,N-dimethylformamide (15mL) The reaction was prepared, and the crude product was separated and purified by silica gel column chromatography (dichloromethane / methanol (v / v)=20 / 1) to obtain the title compound as a white solid (154 mg, 26.2%).
[0323] MS(ESI,pos.ion)m / z:516.3[M+H] + ;
[0324] 1 H NMR (400MHz, DMSO-d 6 )δ(ppm)8.17(s,1H),8.07(s,2H),7.94(s,1H),7.23(d,J=3.3Hz,1H),6.84(d,J=9.1Hz,2H), 6.78(d, J=9.0Hz, 2H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com