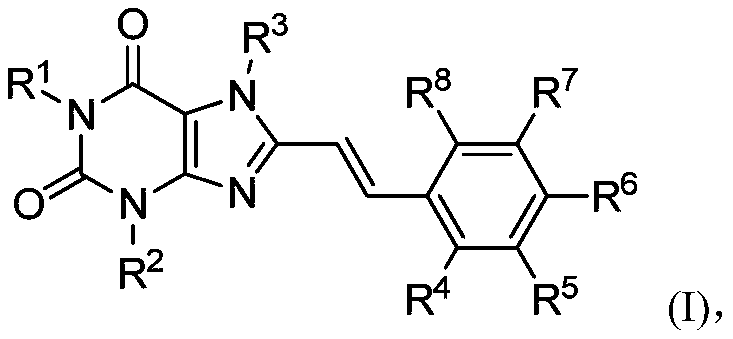
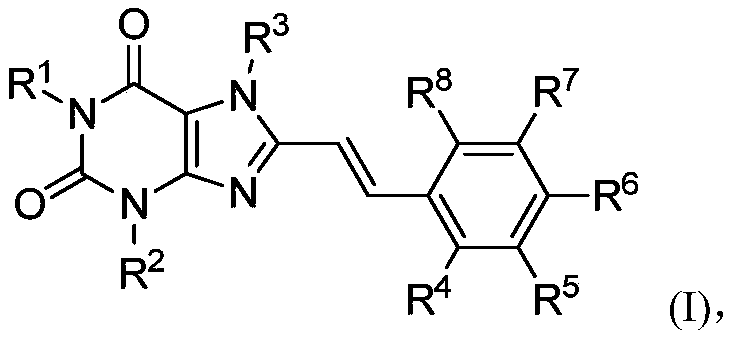
8-substituted styryl xanthine derivative and application thereof
An alkyl and compound technology, used in drug combinations, active ingredients of heterocyclic compounds, cardiovascular system diseases, etc., can solve problems such as adenosine A distribution limitation, achieve good brain/plasma ratio, stable properties, and good bioavailability degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
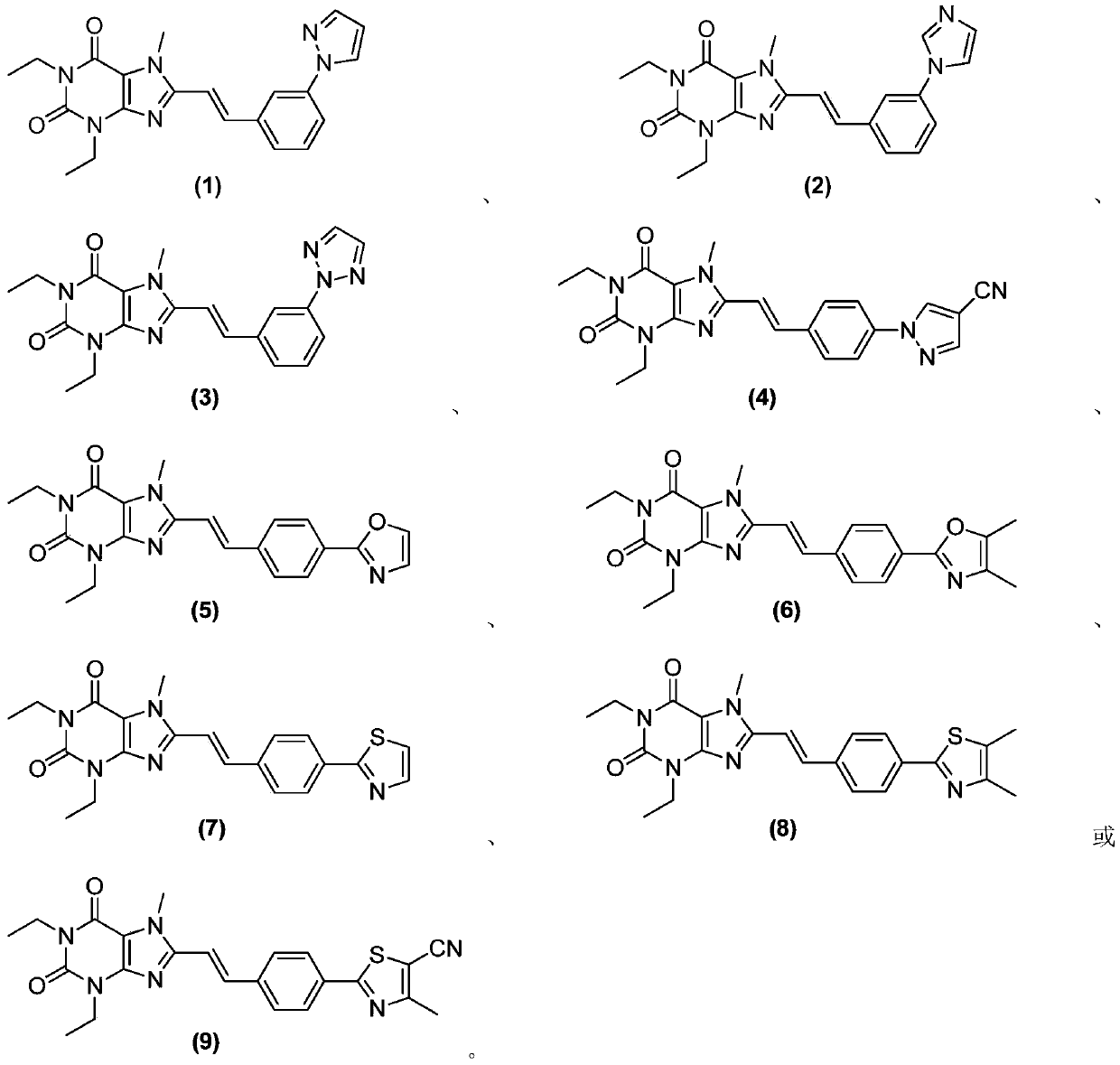
[0291] Example 1 (E)-8-(3-(1H-pyrazol-1-yl)styryl)-1,3-diethyl-7-methyl-1H-purine-2,6(3H, Synthesis of 7H)-diketones
[0292]
[0293] Step 1) (E)-N-(6-amino-1,3-diethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)-3- Synthesis of (3-bromophenyl)acrylamide
[0294]
[0295] Add (E)-3-(3-bromophenyl)acrylic acid (3.3g, 14.5mmol) and dichloromethane (50mL) into a 100mL single-necked round bottom flask at 0°C, add N,N-diisopropyl Ethylamine (7.2mL, 43.6mmol) and HATU (6.6g, 16.8mmol), continue stirring for half an hour; then add 5,6-diamino-1,3-diethylpyrimidine-2,4(1H,3H )-diketone hydrochloride (3.0 g, 12.8 mmol), transferred to 25 ° C for 2 hours. Add water (30mL) and dichloromethane (30mL), separate the layers, collect the organic phase, spin dry under reduced pressure, separate and purify by column chromatography (dichloromethane / methanol (v / v)=20 / 1) to obtain the title compound as White solid (4.3 g, 82.6%).
[0296] MS(ESI,pos.ion)m / z:407.2[M+H] + ;
[0297]...
Embodiment 2
[0312] Example 2 (E)-8-(3-(1H-imidazol-1-yl)styryl)-1,3-diethyl-7-methyl-1H-purine-2,6(3H,7H )-Diketone Synthesis
[0313]
[0314]The title compound of this step was prepared by referring to the method described in step 4 of Example 1, namely (E)-8-(3-bromostyryl)-1,3-diethyl-7-methyl-1H-purine- 2,6(3H,7H)-diketone (300mg, 0.74mmol), imidazole (100mg, 1.47mmol), cesium carbonate (0.4g, 1.23mmol), cuprous iodide (145mg, 0.76mmol), (1R, 2R)-N 1 ,N 2 -Dimethylcyclohexane-1,2-diamine (60mg, 0.42mmol) was prepared by reacting in N,N-dimethylformamide (15mL), and the crude product was separated and purified by silica gel column chromatography (dichloromethane / methanol (v / v)=50 / 1), the title compound was obtained as a yellow solid (178 mg, 61.3%).
[0315] MS(ESI,pos.ion)m / z:391.1[M+H] + ;
[0316] 1 H NMR (600MHz, CDCl 3 )δ (ppm) 7.85 (d, J = 15.7Hz, 2H), 7.62 (d, J = 7.5Hz, 2H), 7.56 (t, J = 7.8Hz, 1H), 7.46–7.41 (m, 2H), 7.02–6.98(m,2H),4.24(q,J=7.1Hz,2H),4.14–4.09(m...
Embodiment 3
[0317] Example 3 (E)-8-(3-(2H-1,2,3-triazol-2-yl)styryl)-1,3-diethyl-7-methyl-1H-purine Synthesis of -2,6(3H,7H)-dione
[0318]
[0319] The title compound of this step was prepared by referring to the method described in step 4 of Example 1, namely (E)-8-(3-bromostyryl)-1,3-diethyl-7-methyl-1H-purine- 2,6(3H,7H)-diketone (0.6g, 1.49mmol), triazole (200mg, 2.9mmol), cesium carbonate (0.73g, 2.24mmol), cuprous iodide (170mg, 0.89mmol), (1R,2R)-N 1 ,N 2 -Dimethylcyclohexane-1,2-diamine (130mg, 0.91mmol) was prepared by reacting in N,N-dimethylformamide (15mL), and the crude product was separated and purified by silica gel column chromatography (petroleum ether / Ethyl acetate (v / v)=10 / 1), the title compound was obtained as a yellow solid (225 mg, 38.6%).
[0320] MS(ESI,pos.ion)m / z:392.1[M+H] + ;
[0321] 1 H NMR (600MHz, CDCl 3 )δ (ppm) 8.36 (s, 1H), 8.11 (d, J = 4.4Hz, 1H), 7.89–7.86 (m, 3H), 7.56 (s, 2H), 7.06 (d, J = 15.6Hz, 1H ), 4.24(q, J=5.9Hz, 2H), 4.11(brs, 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com