Preparation method of nitrendipine
A technology of nitrendipine and reaction solution, which is applied in the field of preparation of nitrendipine, and can solve problems such as inability to overcome the impurities of dimethyl ester and diethyl ester, difficult to control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The embodiment of the present invention provides a preparation method of nitrendipine, comprising:
[0030] Step S11, adding (E)-2-(3-nitrobenzylidene)-3-oxobutanoic acid ethyl ester and 3-aminocrotonate methyl ester into the first solvent, at the first temperature, Reflux reaction, obtains the first reaction solution;
[0031] Step S12, at a second temperature, adding acetic anhydride to the first reaction solution to react to obtain a second reaction solution;
[0032] Step S13, at a third temperature, stirring and reacting the second reaction solution, and filtering to obtain the crude nitrendipine;
[0033] Step S14, recrystallizing the crude nitrendipine to obtain nitrendipine.
[0034] Further, in step S11, the molar ratio of (E)-2-(3-nitrobenzylidene)-3-oxobutanoic acid ethyl ester to the 3-aminocrotonate methyl ester is 1 :1~1:1.5, such as 1:1, 1:1.05, 1:1.1, 1:1.15, 1:1.2, 1:1.25, 1:1.3, 1:1.35, 1:1.4, 1:1.45, 1:1.5, etc., when the molar ratio of (E)-2-(3-n...
Embodiment 1
[0052] Step S201: Add ethyl (E)-2-(3-nitrobenzylidene)-3-oxobutyrate (500.0g, 1.9mol), methyl 3-aminocrotonate (240.3g, 2.1mol ), ethanol (1250ml) was placed in the reaction flask and started to stir, the temperature was raised to 75-80°C, and the reaction was incubated for 1 hour to obtain the first reaction solution;
[0053] Step S202: Add acetic anhydride (9.7 g, 95.0 mmol) to the first reaction solution in step S201, heat it at 75-80°C for 1 hour, and stop heating to obtain a second reaction solution;
[0054] Step S203: the second reaction solution in step S202 was cooled to 15-20°C, stirred at this temperature for 30 minutes, and filtered to obtain crude nitrendipine;
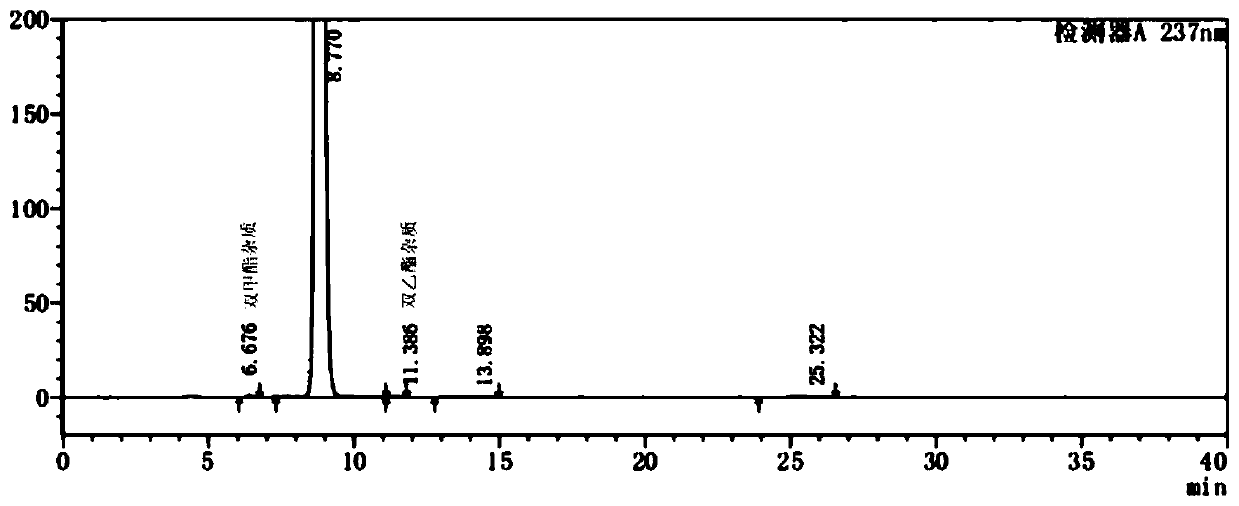
[0055] Step S204: Place the crude nitrendipine in step S203 in ethanol (2000ml), heat up to reflux (about 77°C), dissolve the sample, continue stirring for 30 minutes, filter while hot, and cool the filtrate to 15-20°C with stirring, Filtration, filter cake drying obtains product 575.0g, yield is 84%, H...
Embodiment 2
[0057] Step S301: Add ethyl (E)-2-(3-nitrobenzylidene)-3-oxobutyrate (500.0g, 1.9mol), methyl 3-aminocrotonate (240.3g, 2.1mol ), ethanol (1250ml) was placed in the reaction flask and started to stir, the temperature was raised to 75-80°C, and the reaction was incubated for 1 hour to obtain the first reaction solution;
[0058] Step S302: Add acetic anhydride (19.4 g, 190.0 mmol) to the first reaction solution in step S301, heat at 75-80° C. for 1 hour, and then stop heating to obtain a second reaction solution;
[0059] Step S303: the second reaction solution in step S302 was cooled to 15-20°C, stirred at this temperature for 30 minutes, and filtered to obtain crude nitrendipine;
[0060] Step S304: Place the crude nitrendipine in step S303 in ethanol (2000ml), heat up to reflux (about 77°C), dissolve the sample, continue stirring for 30 minutes, filter while hot, and cool the filtrate to 15-20°C with stirring, Filtration, filter cake drying to obtain product 588.6g, yield 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com