Application of imidazoquinazoline derivatives and salts thereof in preparation of PIM1 enzyme inhibitor, PIM1 enzyme inhibitor and application of PIM1 enzyme inhibitor
A technology of azoquinazolines and derivatives, which is applied in the fields of medicinal chemistry and pharmacotherapeutics, and can solve the problems of easy recurrence, poor chemotherapy sensitivity of myeloma, and difficult clinical treatment of myeloma.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Compounds 1-10 provided by the present invention having the structure represented by formula I were subjected to cell viability experiments.
[0055] Experimental principle: using the commercialized CCK8 kit, the reagent contains WST-8, which is decomposed under the action of the electron carrier 1-methoxy-5-methylphenazinium dimethyl sulfate (1-Methoxy PMS). The dehydrogenase in the cell is reduced to a highly water-soluble yellow formazan product (Formazan dye). The amount of formazan produced is directly proportional to the number of living cells. Therefore, this property can be used directly for cell proliferation and toxicity assays.
[0056] Experimental steps:
[0057] 1. Plating: Experimental group: Inoculate U266 or RPMI-8226 myeloma cells in the logarithmic growth phase in a 96-well plate, inoculate 100 μL of U266 or RPMI-8226 cell suspension in each well, and the number of cells is 1×10 4 / well; the blank control group (Control) only added 100 μL of RPMI-1...
Embodiment 2
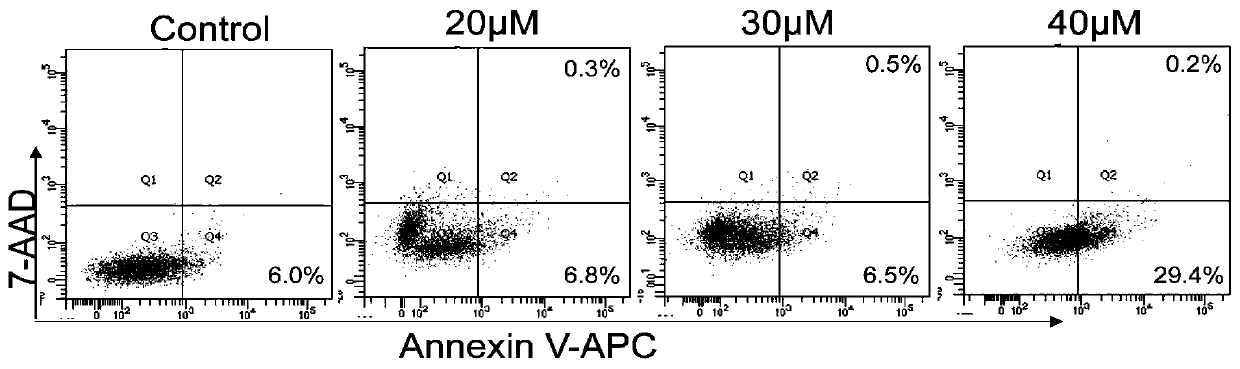
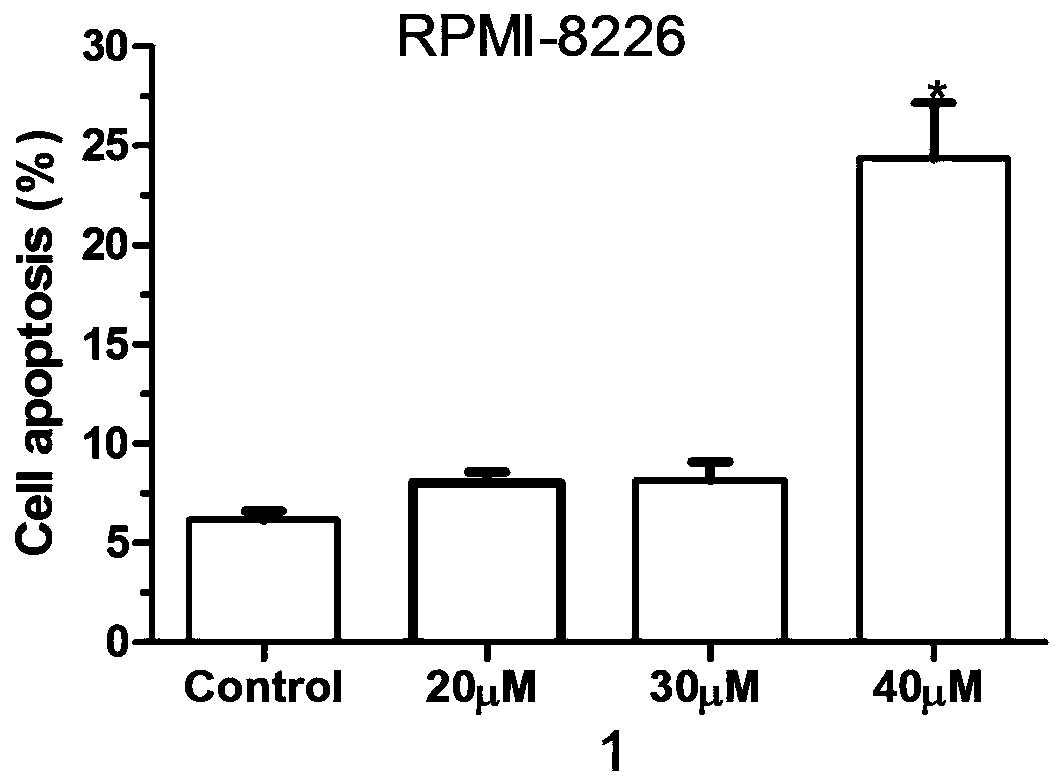
[0065] The compounds 1-10 provided by the present invention having the structure represented by formula I were subjected to cell apoptosis detection research.
[0066] Experimental principle: In normal cells, phosphatidylserine (PS) is only distributed on the inner side of the lipid bilayer of the cell membrane, but in the early stage of apoptosis, the phosphatidylserine (PS) in the cell membrane is turned from the inner side of the lipid membrane to the outer side. Annexin V is Ca with a molecular weight of 35-36kD 2+ Phosphatidylserine-dependent phospholipid-binding protein has a high affinity with phosphatidylserine, so it can bind to the cell membrane of early apoptosis cells through the phosphatidylserine exposed on the outside of the cell. Therefore, Annexin V is used as one of the sensitive indicators for detecting early apoptosis of cells.
[0067] Experimental steps:
[0068] 1. Plating: Take RPMI-8226 cells grown in the logarithmic phase, with 1×10 6 Inoculate a s...
Embodiment 3
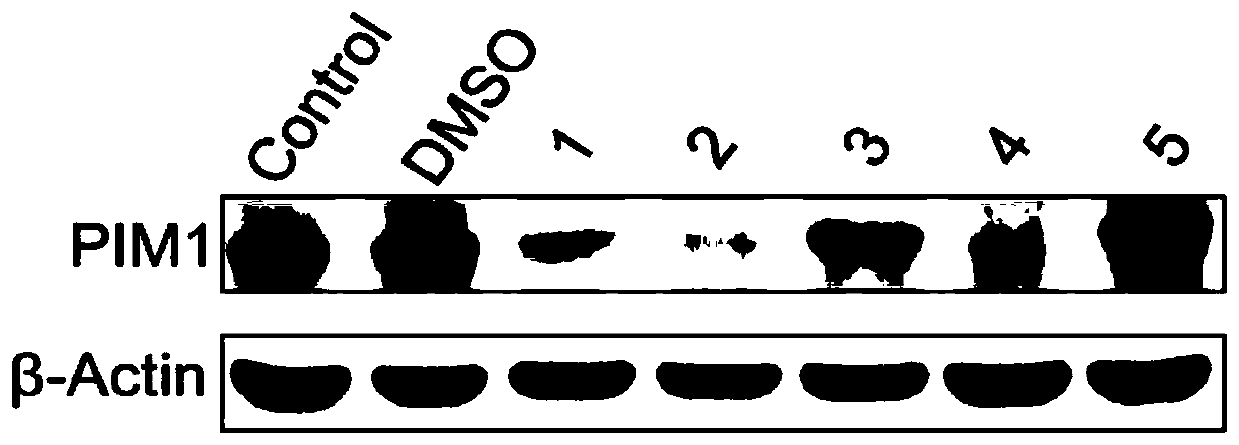
[0075] Compounds 1-10 provided by the present invention were studied by western blot experiment.
[0076] Experimental principle: stain the cells or biological tissue samples treated by gel electrophoresis with specific antibodies. Information about the expression of specific proteins in the analyzed cells or tissues can be obtained by analyzing the location and depth of staining.
[0077] Experimental steps:
[0078] 1. Prepare polyacrylamide gel electrophoresis (SDS-PAGE) gel, plate and collect cell suspension (cell suspension treated with compounds 1-10 respectively), wash twice with PBS 1000rpm, centrifuge for 5min, according to RPMI-8226 cell Add different volumes of RIPA (radioimmunoprecipitation) lysate, lysate at least 30 μL, lyse on ice for 2 min, vortex for 1 min, alternately for 40 min, centrifuge at 4 °C, 5000 rpm for 15 min, collect the supernatant, add etc. Volume 2×SDS loading buffer, boiled for 10min, and stored at -20°C; the blank control group (Control) onl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com