Prussian blue-like derivative and preparation method and application thereof
A Prussian blue-like and Prussian blue-like technology, which is applied in the field of Prussian blue-like derivatives and their preparation, can solve problems such as the inability to effectively improve the electrochemical performance of materials, and achieve the effect of good electrochemical performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] This embodiment provides a Na 1.77 Mn[Fe(CN) 6 ] 0.97 1.73H 2 The preparation method of O, described preparation method comprises the steps:
[0071] 0.97mol of Na 4 Fe(CN) 6 10H 2 O and 1mol of MnSO 4 ·H 2 O mixing, ball milling, the ball milling speed is 1000r / min, the time of ball milling is 0.5h, the ball-to-material ratio of ball milling is 0.5:1, then the ball milling thing obtained is added with water and stirred to control the liquid-solid ratio to be 15mL / g, after stirring The mixed solution was filtered under reduced pressure to obtain the filter residue, then washed three times with deionized water and then dried in vacuum at 80°C for 16h to obtain the Na 1.77 Mn[Fe(CN) 6 ] 0.97 1.73H 2 O.
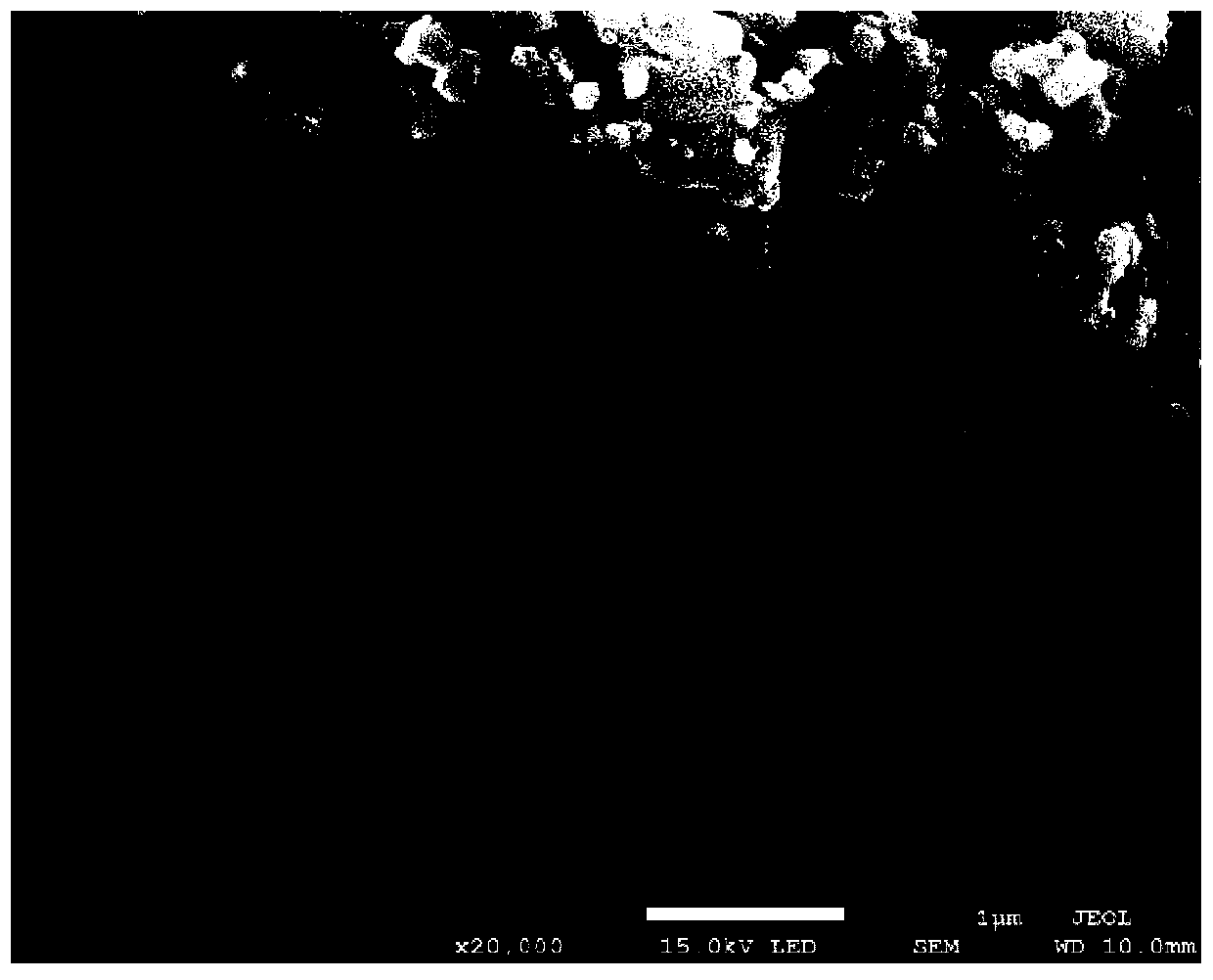
[0072] figure 1 It is the scanning electron micrograph of the class Prussian blue derivative obtained in embodiment 1, from figure 1 It can be seen that the morphology of the obtained product is an aggregate composed of nano-cubic particles, and the size of ...
Embodiment 2
[0077] This embodiment provides a Na 1.93 Mn[Fe(CN) 6 ] 1.07 1.37H 2 The preparation method of O, described preparation method comprises the steps:
[0078] 1.07mol of Na 4 Fe(CN) 6 10H 2 O and 1mol of MnSO 4 Mixing, ball milling, the ball milling speed is 1000r / min, the time of ball milling is 0.5h, the ball-to-material ratio for ball milling is 0.5, then add water to the obtained ball mill and stir to control the liquid-solid ratio to 10mL / g, and the stirred mixed solution vacuum filtration under reduced pressure to obtain the filter residue, then washed three times with deionized water and dried in vacuum at 90°C for 12h to obtain the Na 1.93 Mn[Fe(CN) 6 ] 1.07 1.37H 2 O.
[0079] Figure 6 It is the scanning electron micrograph of the class Prussian blue derivative obtained in embodiment 2, from Figure 6 It can be seen that the morphology of the obtained product is an aggregate composed of nano-cubic particles, and the size of the nano-particles is 100-200nm. ...
Embodiment 3
[0083] This embodiment provides a Na 1.77 Mn[Fe(CN) 6 ] 0.97 1.73H 2 The preparation method of O, described preparation method comprises the steps:
[0084] 0.97mol of Na 4 Fe(CN) 6 10H 2 O and 1mol of MnSO 4 ·H 2 O mixing, ball milling, the ball milling speed is 2000r / min, the time of ball milling is 0.5h, the ball-to-material ratio of ball milling is 0.1, then the ball milling thing obtained is added with water and stirred to control the liquid-solid ratio to be 10mL / g, and the mixed The liquid was filtered under reduced pressure to obtain the filter residue, then washed three times with deionized water and then dried in vacuum at 60°C for 30h to obtain the Na 1.77 Mn[Fe(CN) 6 ] 0.97 1.73H 2 O.
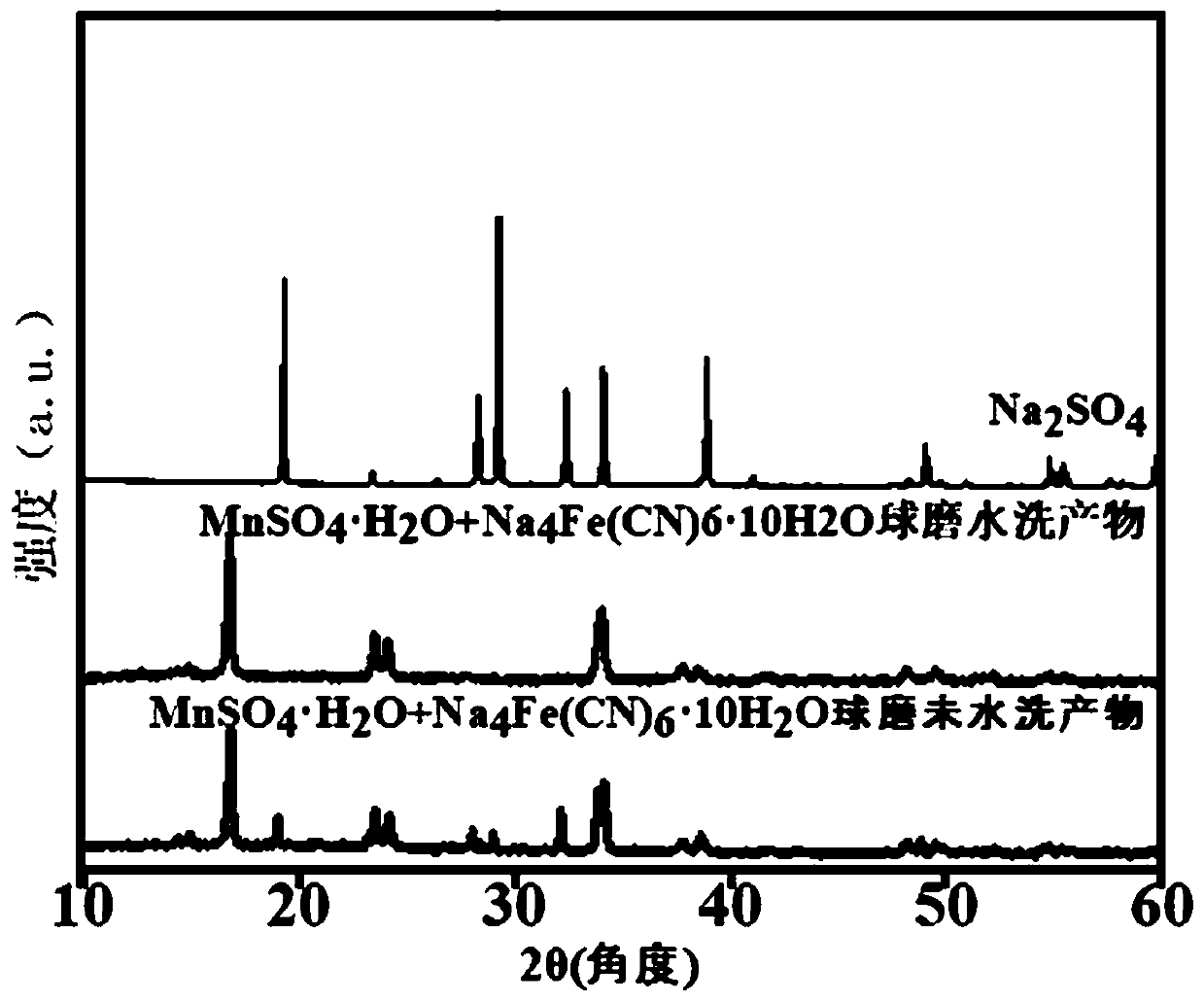
[0085] Scanning electron microscopy and XRD tests of this example show that the Prussian blue-like derivative obtained is the same as the Prussian blue-like derivative obtained in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com