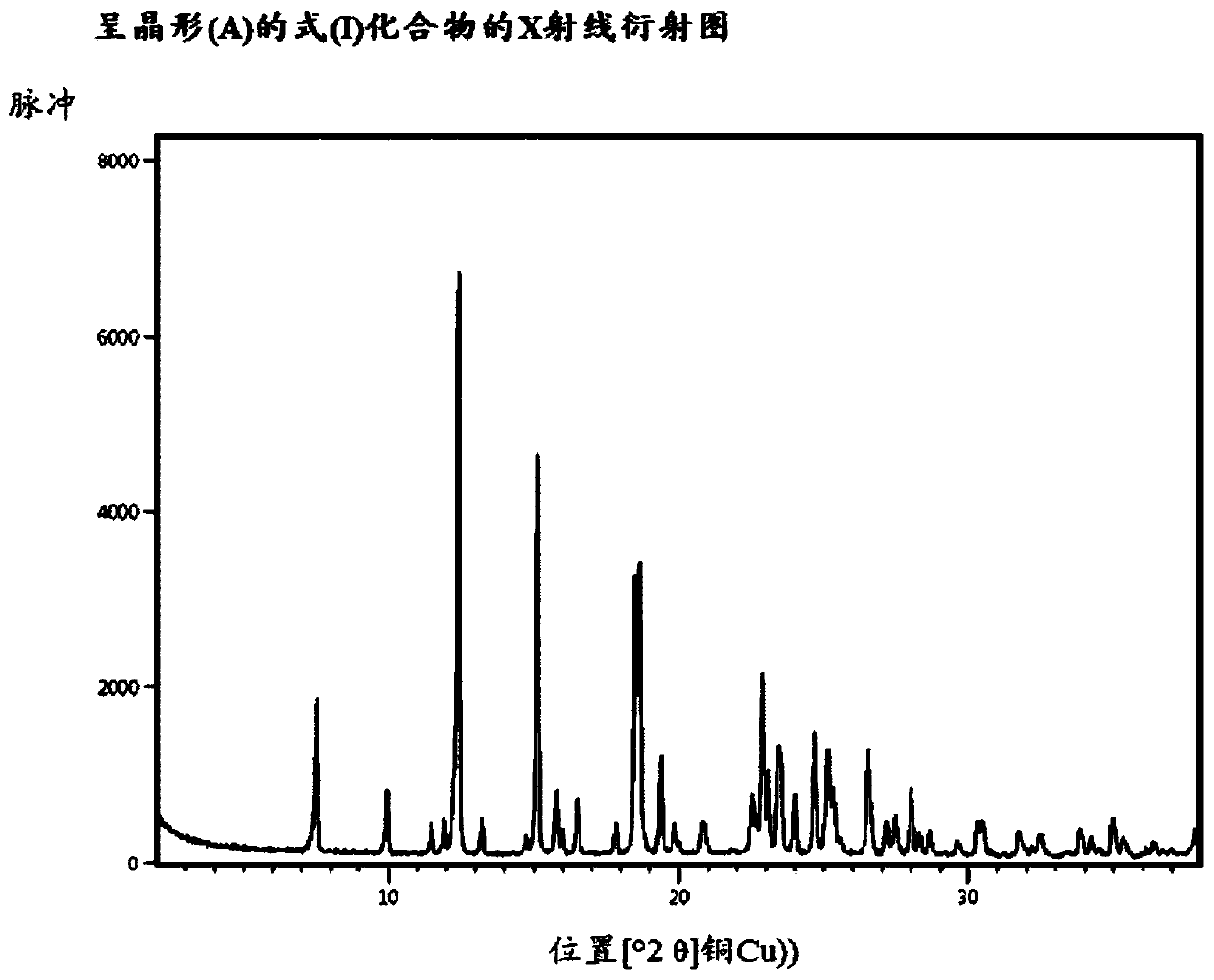
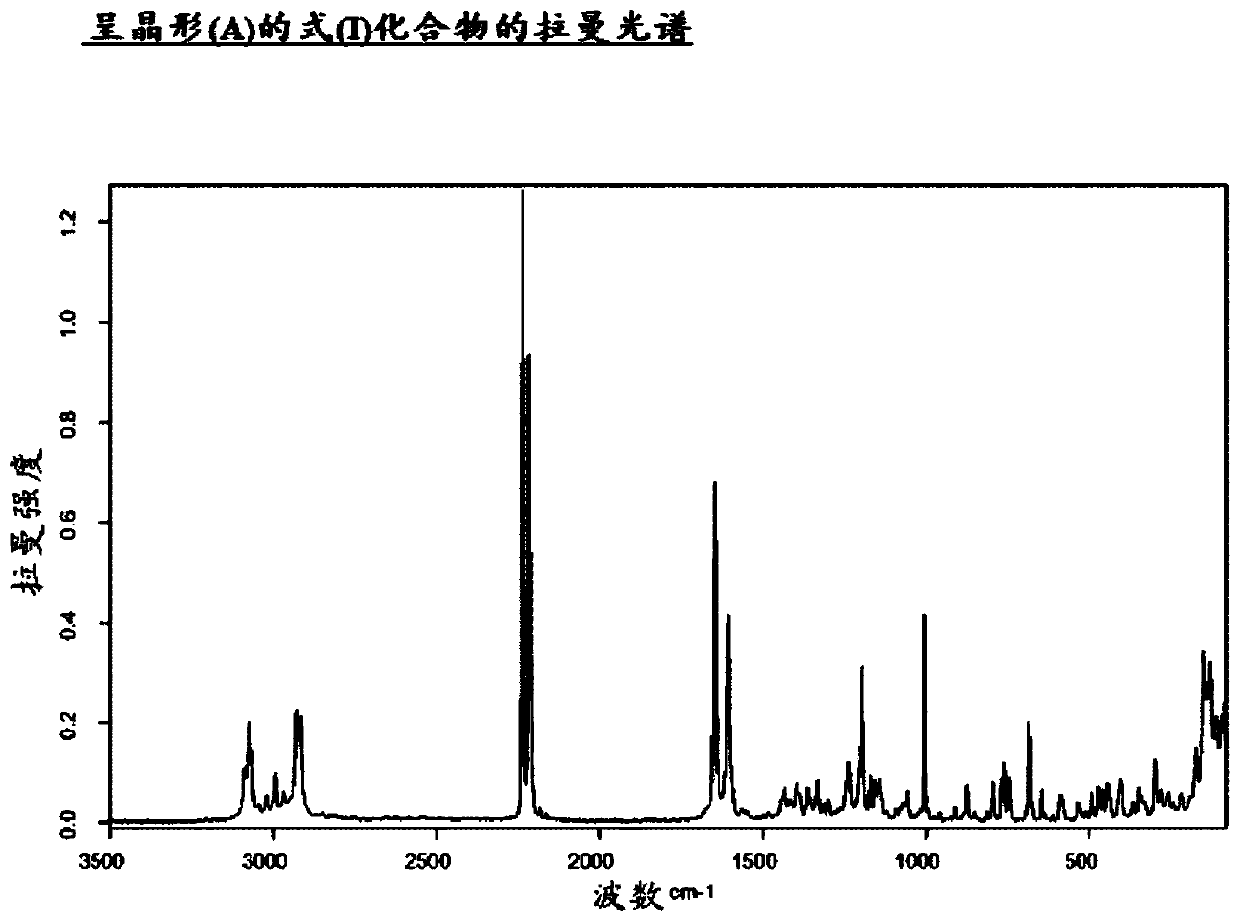
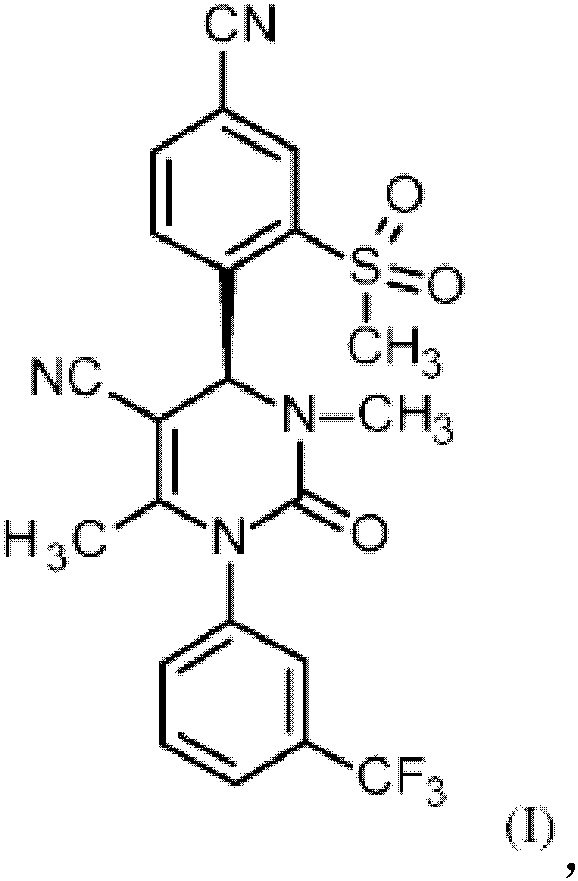
Method for producing compound
A compound, a technology for wound healing, used in the production of pharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0203] 4-Formyl-3-fluorobenzonitrile (XV)
[0204] 400 g (1.97 mol) of 4-bromo-2-fluorobenzaldehyde (XIV) as a solution in 2.01 DMF were mixed with 183 g (0.433 mol) of potassium hexacyanoferrate (K 4 [Fe(CN) 6 ]) and 165.5 g (1.97 mol) of sodium bicarbonate were combined, and 2.2 g (9.85 mmol) of palladium acetate were added. The resulting product was stirred at 120°C for 2.5 hours. Allow to cool to 20°C, then add 2.01 water to the batch. Extraction was performed with 4.01 MtBE, and the aqueous phase was washed again with 1.51 MtBE. The organic phases were combined and mixed with 21 water. Most of the MtBE was distilled off at 30 °C in slight vacuum. The product crystallized out. It was cooled to 3°C and stirred at this temperature for 1 hour. The product was filtered off and washed again with water (twice 0.81 each). Drying was carried out in vacuo at 40°C. Yield: 241 g (80% of theory) of beige solid.
[0205] MS(EIpos): m / z=150[M+H]+
[0206] 1H-NMR (400MHz, DMSO...
Embodiment 2
[0208] 4-Formyl-3-methylsulfonylbenzonitrile (VI)
[0209] 200 g (1.34 mol) of 4-formyl-2-fluorobenzonitrile (XV) were provided as a solution in 0.81 DMSO, and 192 g (1.88 mol) of the sodium salt of methanesulfinic acid were added. It was stirred at 50°C for 4 hours. The resulting product was cooled to 20°C. The reaction mixture was added to 8.01 water. The product crystallized out. It was stirred at room temperature for 1 hour. The product was filtered off and washed with water (2 times 0.11 each). Drying was carried out in vacuo at 40°C. Yield: 256 g (91% of theory) of beige solid.
[0210] MS(ESIpos): m / z(%)=191.1(15)[M-18] + , 161.0 (100).
[0211] 1H-NMR (400MHz, DMSO-d6): δ=3.57(s, 3H), 8.10(d, 1H), 8.38(d, 1H), 8.45(s, 1H), 10.62(s, 1H).
Embodiment 3
[0213] (racemic)-4-[4-cyano-2-(methylsulfonyl)phenyl]-6-methyl-2-oxo-1-[3-trifluoromethyl]phenyl]- Allyl 1,2,3,4-tetrahydropyrimidine-5-carboxylate (IX)
[0214] To triethyl phosphate (124.3 g, 683 mmol) was added phosphorus pentoxide (64.6 g, 455 mmol) in 3 portions at 20 °C, and the resulting product was stirred at 40 °C for 3 h. It was then diluted with THF (115ml), stirred at 20°C for 30min, and 4-formyl-3-(methylsulfonyl)benzonitrile (VI) (119g, 569mmol) and 1-[3-(tri Fluoromethyl)phenyl]urea (VII) (116 g, 569 mmol). Thereafter, allyl (VIII) acetoacetate (121 g, 852 mmol) was partitioned for 20 min, at which time the temperature was increased to around 60°C. The mixture was stirred at 80 °C for 4 h. For work-up, water (115 ml) was added at 40°C and it was stirred at 25°C for 30 min. The product was filtered off and washed with water (280ml). The residue was stirred with MtBE (280ml) for 20min, filtered off again and washed with MtBE (220ml). Drying was carried out i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com