Nano solid electrolyte, preparation method thereof and lithium ion battery
A solid electrolyte, nanotechnology, applied in secondary batteries, circuits, electrical components, etc., can solve the problems of uneven particle size distribution, large differences between particles, and increase in product particle size, and achieve regular morphology. order, prevent growth, and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
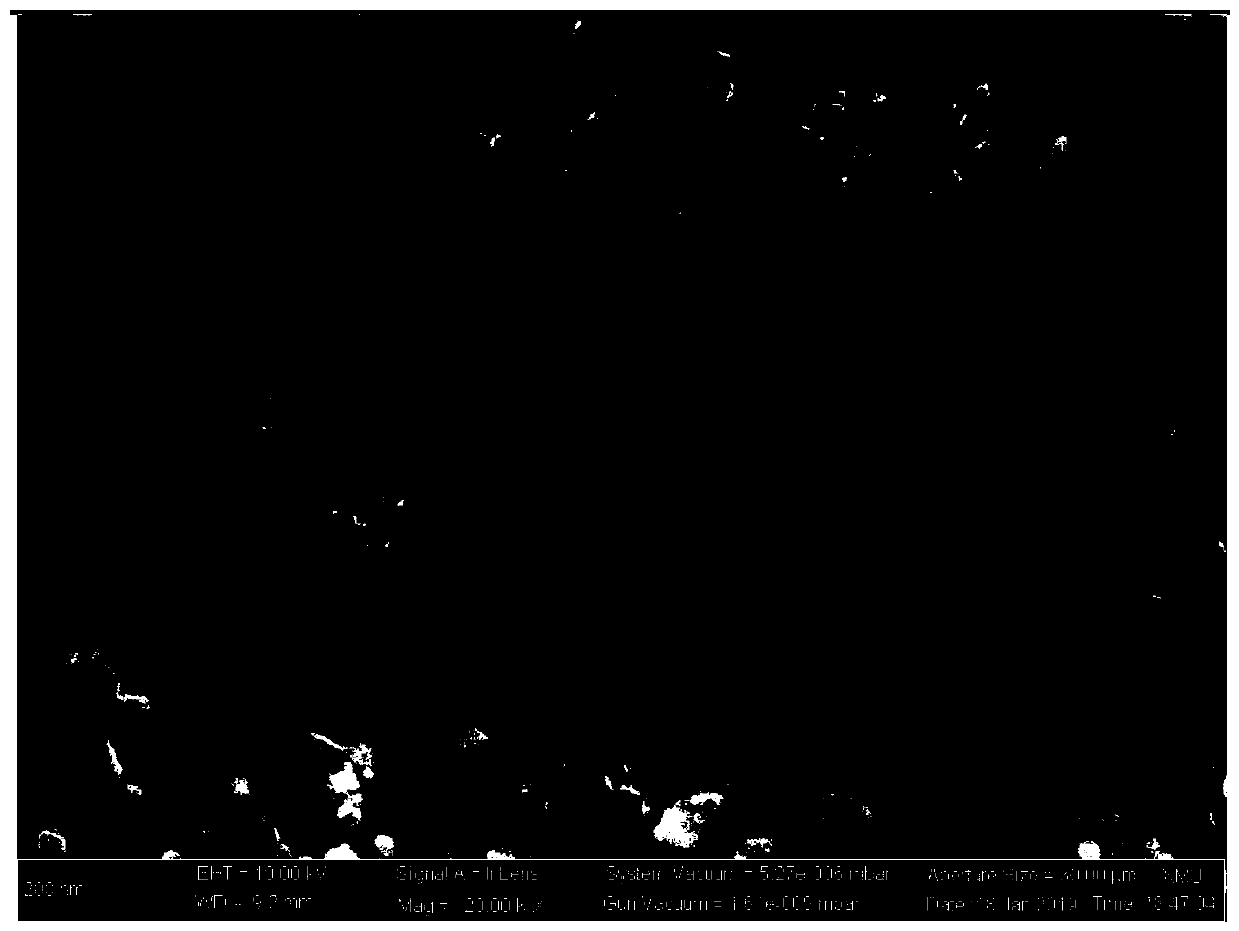
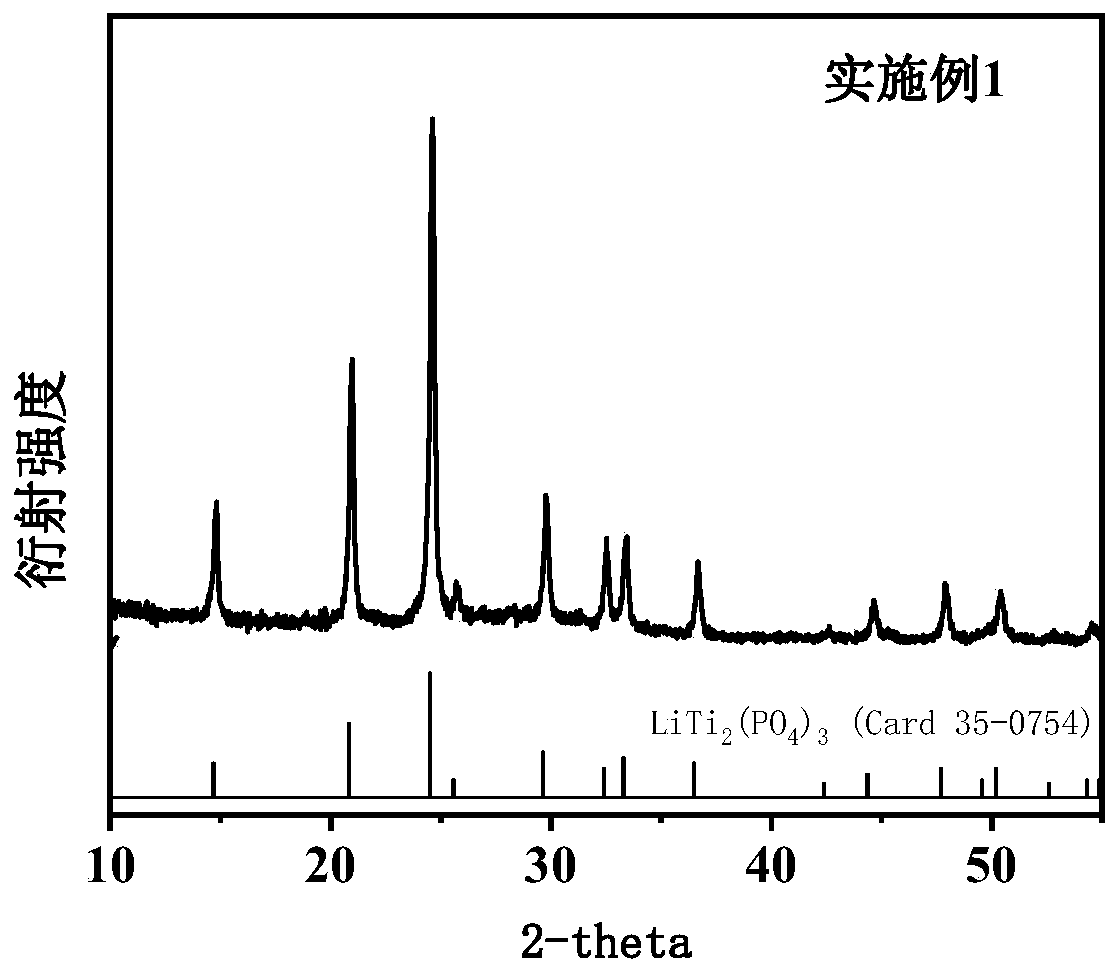
Image
Examples
Embodiment 1
[0048] 70 ml of deionized water was added to a 150 ml beaker, and 14.25 g of butyl titanate was added dropwise during stirring to form a precipitate. After filtering the precipitate with a suction filter, transfer the precipitate to a 200ml beaker, add 30ml of deionized water, 7ml of concentrated nitric acid and 17.20g of citric acid respectively, and stir to obtain a clear solution. Add 2.52 grams of lithium nitrate and 3.93 grams of aluminum nitrate to the clear solution respectively, and stir until completely dissolved to obtain a mixed solution of metal ions. In another 100ml beaker, 9.03 g of ammonium dihydrogen phosphate and 40 ml of deionized water were dissolved to obtain an ammonium dihydrogen phosphate solution, and the ammonium dihydrogen phosphate solution was added dropwise to the above metal ion mixed solution to form a sol. After the sol was stirred for 30 minutes, 18.6 grams of acrylamide, 7.44 grams of N,N-dimethylacrylamide and 3.00 grams of ammonium persulfa...
Embodiment 2
[0051] 70 ml of deionized water was added to a 150 ml beaker, and 14.25 g of butyl titanate was added dropwise during stirring to form a precipitate. After filtering the precipitate with a suction filter, transfer the precipitate to a 200ml beaker, add 30ml of deionized water, 7ml of concentrated nitric acid and 8.06g of oxalic acid, and stir to obtain a clear solution. Add 2.52 grams of lithium nitrate and 3.93 grams of aluminum nitrate to the clear solution respectively, and stir until completely dissolved to obtain a mixed solution of metal ions. In another 100ml beaker, 9.03 g of ammonium dihydrogen phosphate and 40 ml of deionized water were dissolved to obtain an ammonium dihydrogen phosphate solution, and the ammonium dihydrogen phosphate solution was added dropwise to the above metal ion mixed solution to form a sol. After the sol was stirred for 30 minutes, 18.6 grams of acrylamide, 7.44 grams of N,N-dimethylacrylamide and 3.00 grams of ammonium persulfate were added ...
Embodiment 3
[0053] 70 ml of deionized water was added to a 150 ml beaker, and 14.25 g of butyl titanate was added dropwise during stirring to form a precipitate. After filtering the precipitate with a suction filter, transfer the precipitate to a 200ml beaker, add 30ml of deionized water, 7ml of concentrated nitric acid and 17.20g of citric acid respectively, and stir to obtain a clear solution. Add 2.52 grams of lithium nitrate and 3.93 grams of aluminum nitrate to the clear solution respectively, and stir until completely dissolved to obtain a mixed solution of metal ions. In another 100ml beaker, 9.03 g of ammonium dihydrogen phosphate and 40 ml of deionized water were dissolved to obtain an ammonium dihydrogen phosphate solution, and the ammonium dihydrogen phosphate solution was added dropwise to the above metal ion mixed solution to form a sol. After the sol was stirred for 30 minutes, 18.6 grams of acrylamide, 7.44 grams of N,N-dimethylacrylamide and 3.00 grams of ammonium persulfa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com