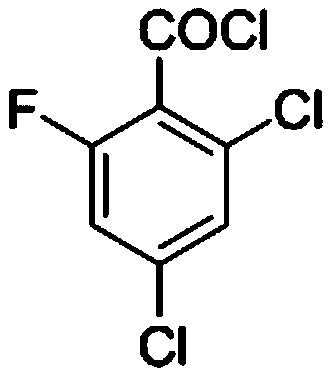
Method for synthesizing 2, 4-dichloro-6-fluorobenzoyl chloride by adopting two-step chlorination process
A technology of fluorobenzoyl chloride and two-step chlorination method, applied in chemical instruments and methods, acyl halide preparation, organic chemistry and other directions, can solve the problems of difficult source of fluorobenzoic acid, difficult to guarantee product quality, difficult to achieve synthesis method and the like , to solve the problems of quality and impurity control, accurate chlorination position, and high product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
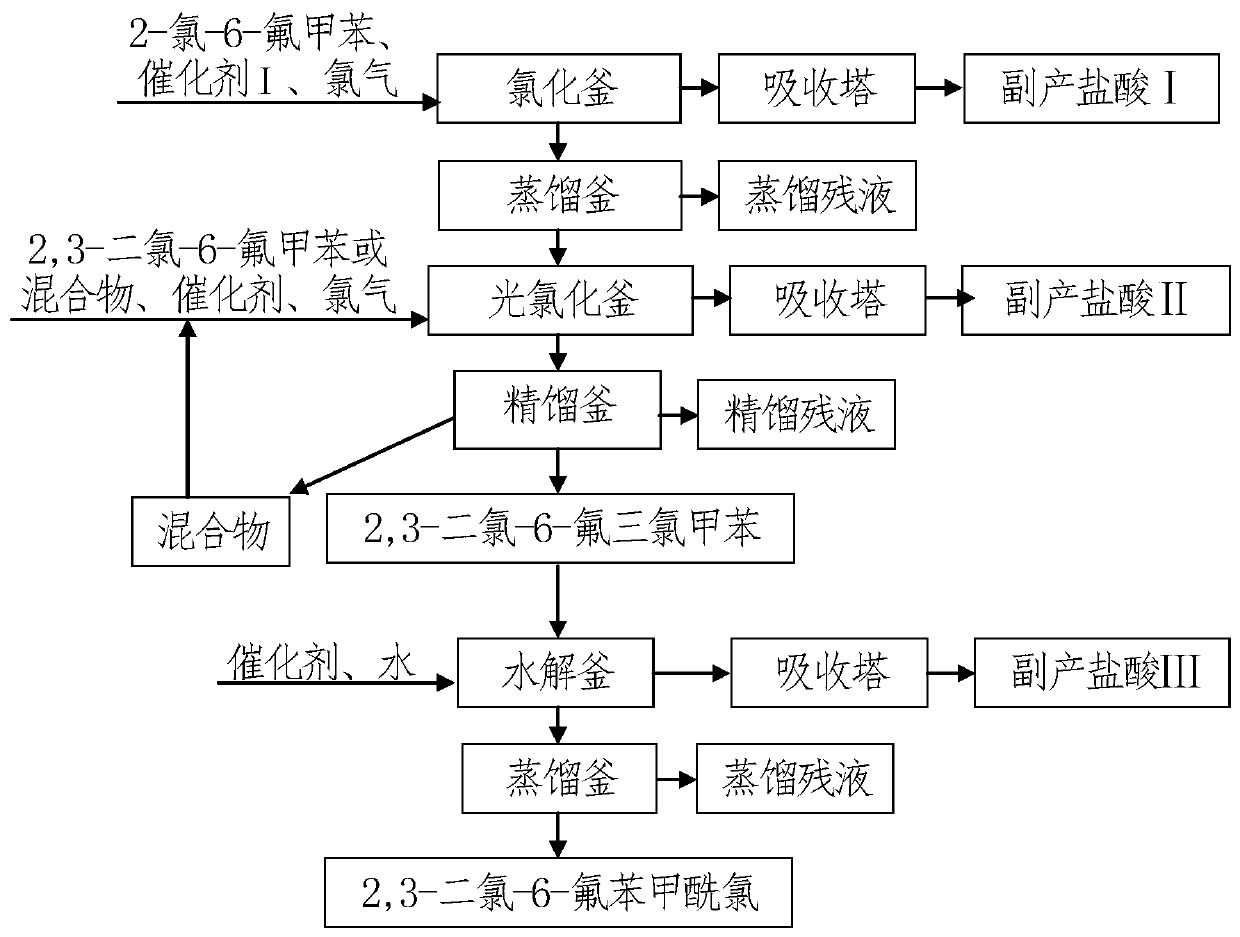
[0053] Embodiment 1, a method for synthesizing 2,4-dichloro-6-fluorobenzoyl chloride by a two-step chlorination method, the following steps are carried out successively:
[0054] 1), put 2000 kilograms of 2-chloro-6-fluorotoluene (content 99.5%) and 2 kilograms of catalyst ferric chloride into the chlorination kettle, feed chlorine gas after heating up to 80°C, and keep warm at 80-90°C For the chlorination reaction, the total amount of chlorine gas introduced is about 1081 kg; the reaction time is 6 hours.
[0055]After the above-mentioned reaction time arrives, turn off chlorine to stop the reaction, and detect through gas chromatography, raw material remains only 0.3% (that is, more than 99.5% is converted into 2,4-dichloro-6-fluorotoluene); Open the vacuum on the chlorination kettle The valve is degassed for 30 minutes to obtain the chlorinated product I and tail gas I respectively; the tail gas I (hydrogen chloride) is sprayed and circulated with about 1200L of water to ab...
Embodiment 2
[0070] Embodiment 2, a method for synthesizing 2,4-dichloro-6-fluorobenzoyl chloride by a two-step chlorination method, the step 5) of embodiment 1 is increased to 164.6 kilograms of water from 159.7 kilograms, and others are equivalent to implementing Example 1, the details are as follows:
[0071] Step 1) to step 4) are the same as step 1) to step 4) in Example 1.
[0072] 5), 2646.5 kg of 2,4-dichloro-6-fluorobenzotrichloride obtained from rectification in step 4) was put into the reaction kettle, and 13.3 kg of catalyst anhydrous zinc chloride was put into it, and the temperature was raised by steaming, when the temperature of the kettle rose to 140°C Start to slowly add 164.6 kg of water, and control the rate of addition, so that the entire time of adding water is 10 hours to obtain a hydrolyzate; the temperature is controlled to be (140±10)°C during the entire hydrolysis process.
[0073] After the tail gas III produced by hydrolysis is cooled by the condenser, it is sp...
Embodiment 3
[0076] Embodiment 3, a method for synthesizing 2,4-dichloro-6-fluorobenzoyl chloride by a two-step chlorination method, replace part with 1077 kilograms of rectification gained mixture (front fraction) in step 4) in embodiment 1 2,4-dichloro-6-fluorotoluene returns to step 3) to carry out photochlorination, and others are equal to embodiment 1, specifically as follows:
[0077] Step 1) to step 2) are the same as step 1) to step 2) in Example 1.
[0078] 3), 1077 kilograms of the mixture (front fraction) gained from rectification in step 4) of Example 1 and 1423 kilograms of 2,4-dichloro-6-fluorotoluene obtained in step 2) are transferred to the photochlorination kettle together, and Catalyst phosphorus trichloride 5 kg, turn on the ultraviolet lamp, pass steam and heat up to 100°C, then start to pass chlorine gas to carry out photochlorination reaction, the reaction temperature is 100-110°C, the amount of chlorine gas introduced is about 1876 kg; the reaction time is 16h;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com