Application of cobratide preparation in preparation of medicine for treating haemorrhoids
A technology of Kebo peptide and preparation, applied in the field of drug application, can solve the problems of Kebo peptide hemorrhoids that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0028] Experimental example 1 Acetic acid-induced pain writhing experiment
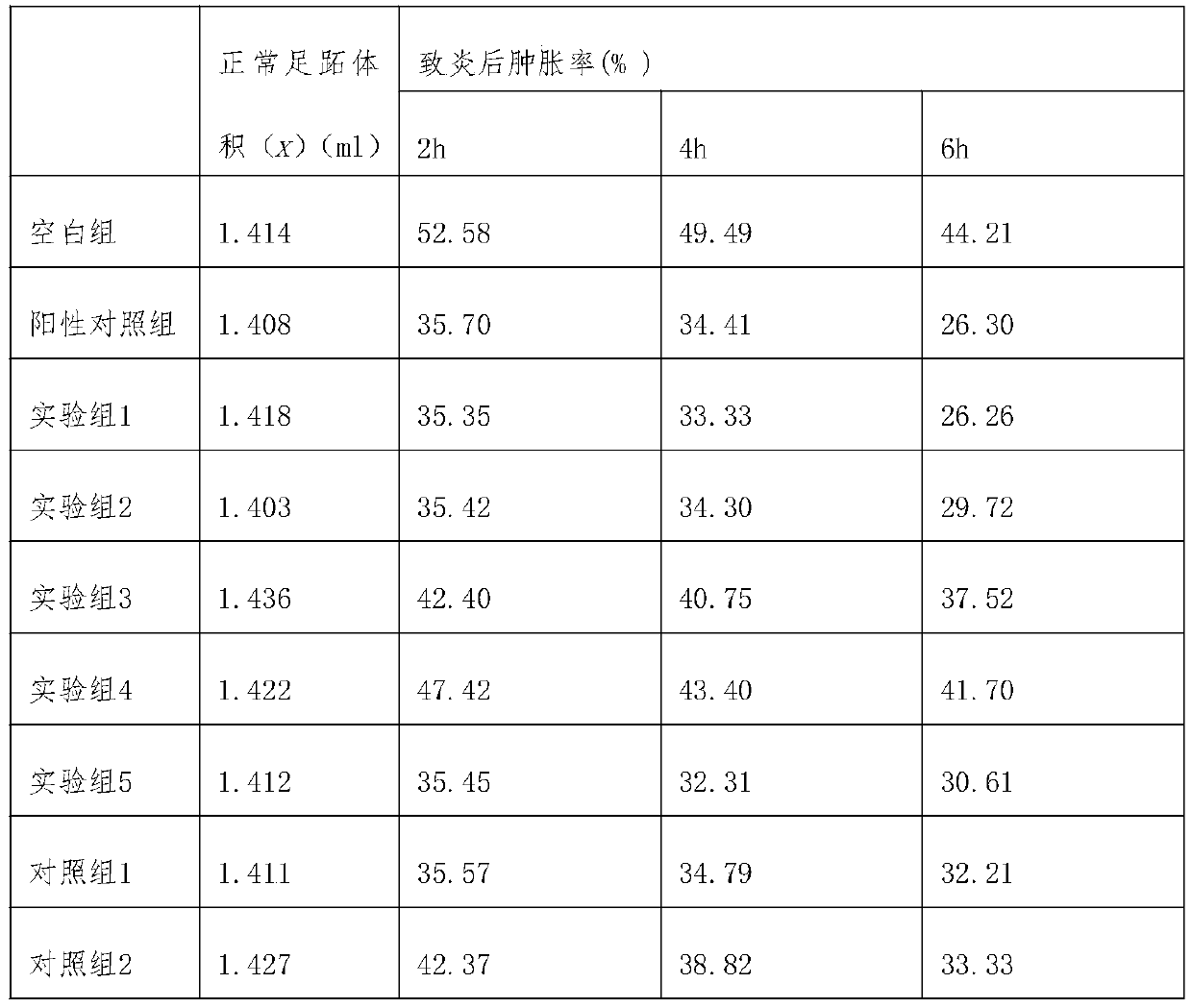
[0029] 1. Experimental objects: 100 SPF grade Kunming mice, half male and half male, weighing 18-22g.
[0030]2. Experimental method: 100 mice were randomly divided into experimental group 1-5, comparison group 1-3, positive control group (Xiaozhiling injection), and blank control group, with 10 mice in each group, half male and half male; experiment The mice in group 1 were injected intramuscularly with Cobotide injection, 0.57ml once a day, twice a day, and the time between the two injections was longer than 6 hours; the mice in experimental group 2 were given Kobotide oral tablets, once a day, 0.018g each time; Experimental group 3 was fed with Cobotide enteric-coated tablets, once a day, 0.018g each time; Experimental group 4 mice were rectally administered Kobotide suppositories, 2 times a day, 0.28g each time ; In the experimental group 5, the mice were administered to the oral mucosa, and the ...
experiment example 2
[0034] Experimental example 2 hemostasis experiment
[0035] 1. The experimental object is the same as the experimental example 1
[0036] 2. Experimental method: the administration method is the same as that of Experimental Example 1. One hour after the last administration, the coagulation time and bleeding time of each mouse were measured by capillary method and tail-cutting method. Insert a capillary glass tube with an inner diameter of 1 mm into the venous plexus of the mouse medial canthus to collect blood, so that the blood column in the capillary glass tube reaches 5 cm, break a small section of glass tube every 30 s, and observe whether there is blood coagulation thread, from the blood collection to the appearance The time of hemagglutination is the coagulation time. Put the mouse in the holder, cut off the tail with sharp scissors from 1.5cm from the tip of the tail, count the time when the blood overflows by itself, absorb the blood with filter paper every 30s, and...
experiment example 3
[0040] Experimental example 3 Influence test of mouse peritonitis exudation caused by acetic acid
[0041] 1. The experimental object is the same as the experimental example 1
[0042] 2. Experimental method: the administration method is the same as that of Experimental Example 1. 1 hour after the last administration, each mouse was injected with 0.5% Evans blue 0.1ml / mouse into the tail vein, and 0.6% acetic acid solution 0.1ml / 10g was injected intraperitoneally 10 minutes later, and then killed by decapitation 20 minutes after the injection, the abdominal cavity was washed with normal saline, and all the mice were collected and washed. After centrifugation, take the supernatant and measure its OD value at a wavelength of 578nm in a semi-automatic biochemical analyzer. The OD value represents the concentration of Evans blue in the washing liquid, which indirectly reflects the degree of peritonitis exudation; the results are shown in Table 3 Show:
[0043] table 3
[0044] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com