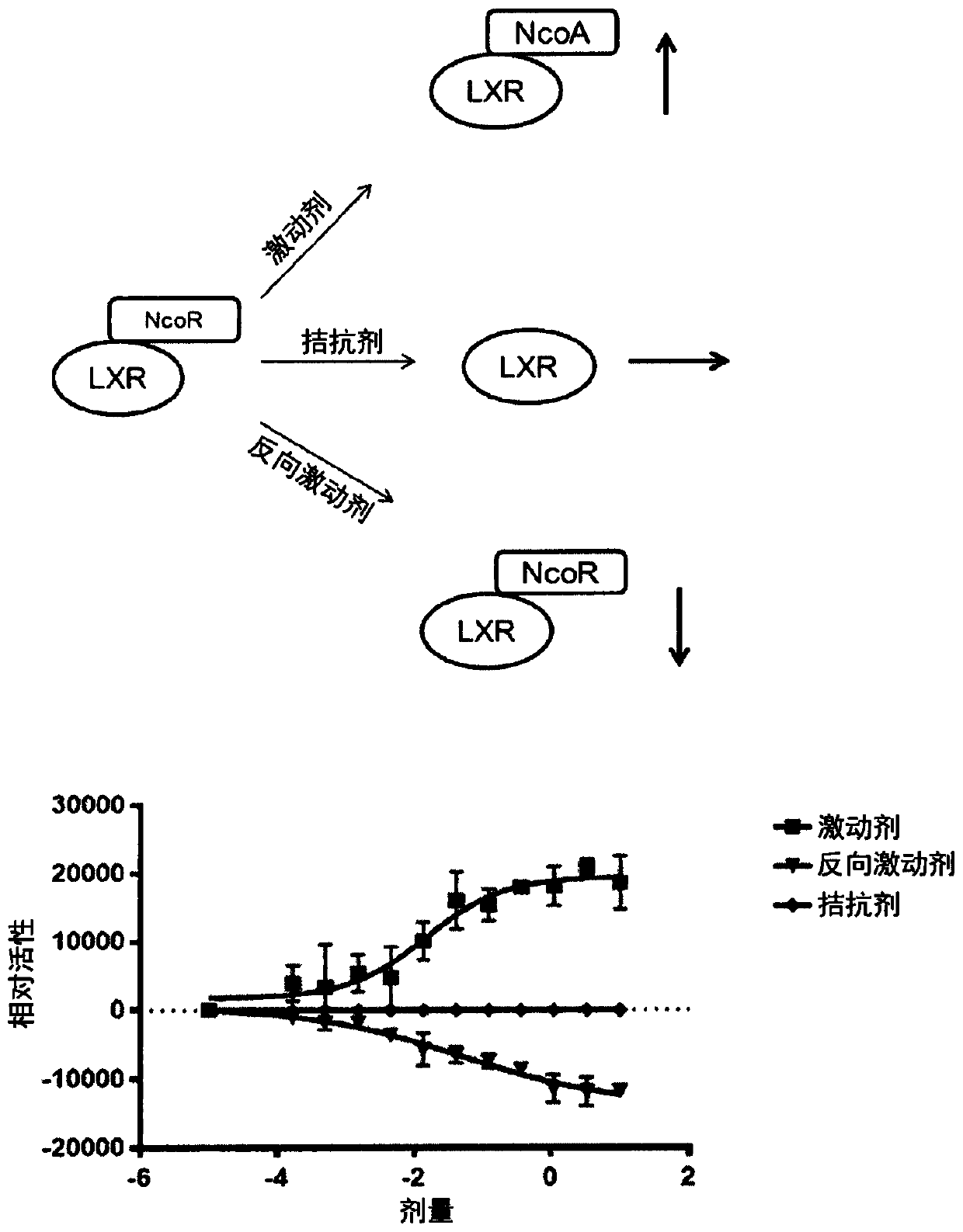
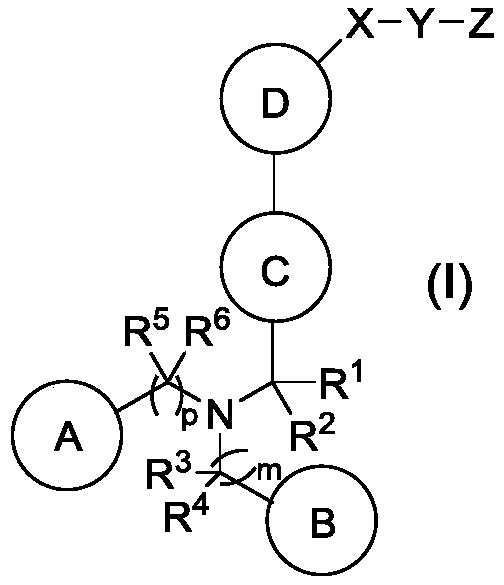
Amine or (THIO)amide containing lxr modulators
A technology of substituents and oxo groups, applied in the field of new compounds, can solve problems such as inactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example P1
[0424]
[0425] Step 1: (4-Bromo-2-mercaptophenyl)methanol (P1a)
[0426]
[0427] To a solution of 4-bromo-2-mercaptobenzoic acid (1.50 g, 6.50 mmol) in THF (30 mL) was added BH 3 (13 mL, 1 M in THF). The mixture was stirred overnight and quenched with water (30 mL). EA (20 mL) was added and the organic layer was separated and the aqueous layer was washed with EA (3 x 20 mL). The combined organic layers were washed with brine (30 mL), washed with Na 2 SO 4 Drying and concentration afforded Compound P1a as a yellow solid.
[0428] Step 2: Ethyl 2-((5-bromo-2-(hydroxymethyl)phenyl)thio)acetate (P1b)
[0429]
[0430] To a mixture of compound P1a (436 mg, 2.00 mmol) and ethyl 2-bromoacetate (306 mg, 2.00 mmol) in DMF (10 mL) was added Cs 2 CO 3 (2.0 g, 6.0 mmol), and the mixture was stirred overnight, diluted with water (100 mL), and extracted with EA (3 x 30 mL). The combined organic layers were washed with brine (30 mL), washed with Na 2 SO 4 Drying, con...
preparation example P2
[0434]
[0435] Step 1: N-(4-Bromobenzyl)-2-mesitylethan-1-amine (P2a)
[0436]
[0437] A solution of 2-mesitylethan-l-amine (300 mg, 1.84 mmol) and 4-bromobenzaldehyde (339 mg, 1.84 mmol) in MeOH (30 mL) was stirred at room temperature overnight. After adding NaBH 4 (105 mg, 2.76 mmol), the mixture was stirred overnight at room temperature, diluted with water, adjusted to pH~11 by addition of 1N NaOH, concentrated and extracted with EA (3x). The combined organic layers were washed with water and brine, washed with Na 2 SO 4 Drying, filtration and concentration afforded compound P2a as a yellow oil.
[0438] Step 2: N-(4-bromobenzyl)-2-mesityl-N-((5-(trifluoromethyl)furan-2-yl)methyl)ethane-1- Amine (P2)
[0439] Compound P2a (724mg, 2.19mmol), 2-(bromomethyl)-5-(trifluoromethyl)furan (499mg, 2.19mmol) and K 2 CO 3 (604 mg, 4.37 mmol) in ACN (40 mL) was added KI (363 mg, 2.19 mmol). The mixture was stirred at 80 °C overnight, cooled, filtered, concentrated ...
preparation example P3
[0444]
[0445] Step 1: tert-butyl 4-bromo-2,6-difluorobenzoate (P3a)
[0446]
[0447] 4-bromo-2,6-difluorobenzoic acid (25.0g, 110mmol), Boc2 A mixture of O (50.0 g, 242 mmol) and DMAP (1.3 g, 11 mmol) in tert-BuOH (200 mL) was stirred overnight at 40 °C, concentrated and purified by FCC (PE:EA=50:1) to give Compound P3a as a yellow oil. MS: 292(M+1) + .
[0448] Step 2: tert-butyl 4-bromo-2-fluoro-6-((2-methoxy-2-oxoethyl)thio)benzoate (P3b)
[0449]
[0450] To a solution of methyl 2-mercaptoacetate (11.2 g, 106 mmol) in anhydrous DMF (50 mL) was added NaH (60%, 5.1 g, 130 mmol) at 0°C. The mixture was stirred for 30 min. The mixture was then added to a solution of compound P3a (31 g, 106 mmol) in anhydrous DMF (100 mL). The mixture was stirred at room temperature for 2 h, washed with H 2 Diluted with O (1000 mL) and extracted with EA (3x). The combined organic layers were washed with H 2 Washed with O and brine, concentrated and purified by FCC (PE:EA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com