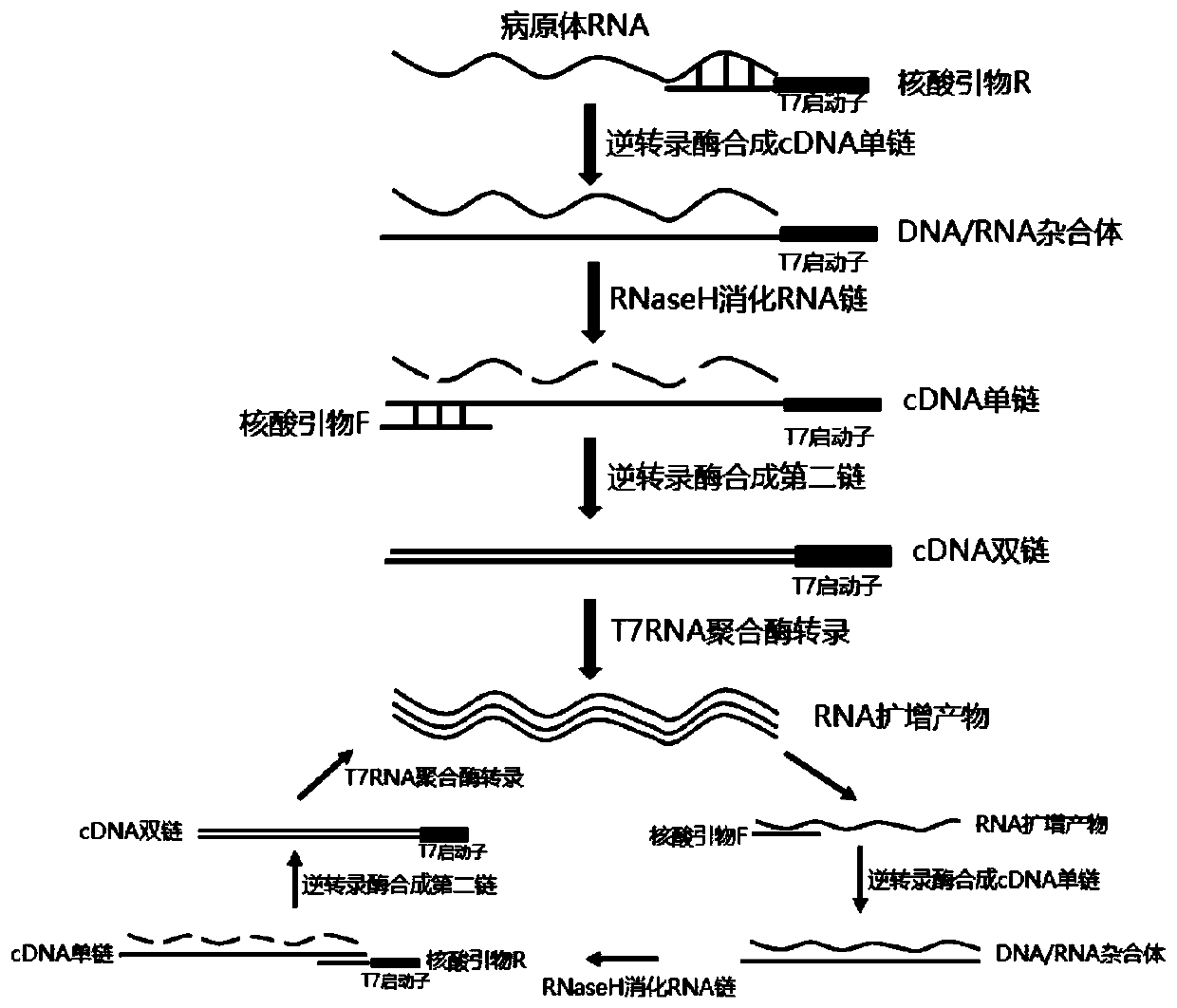
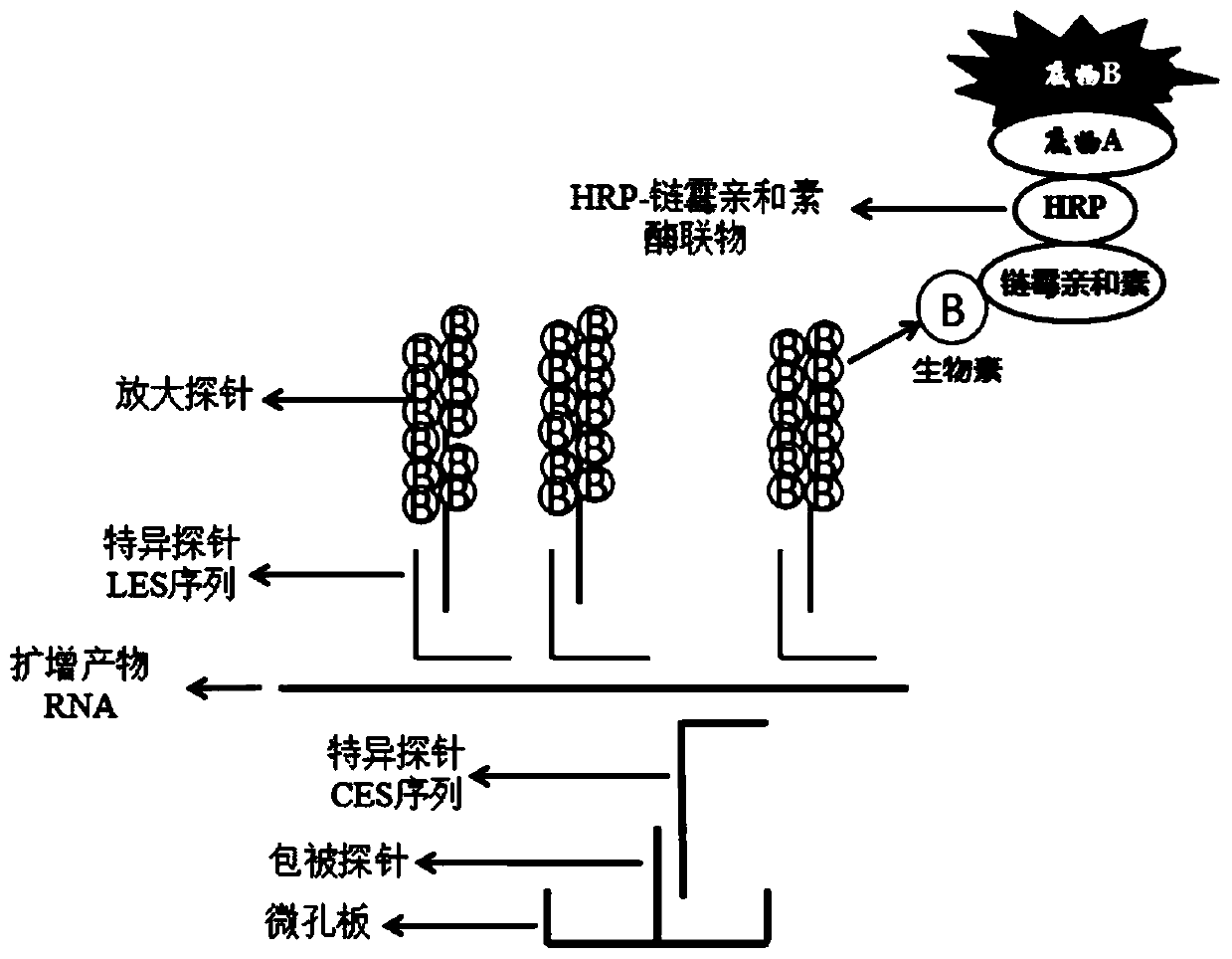
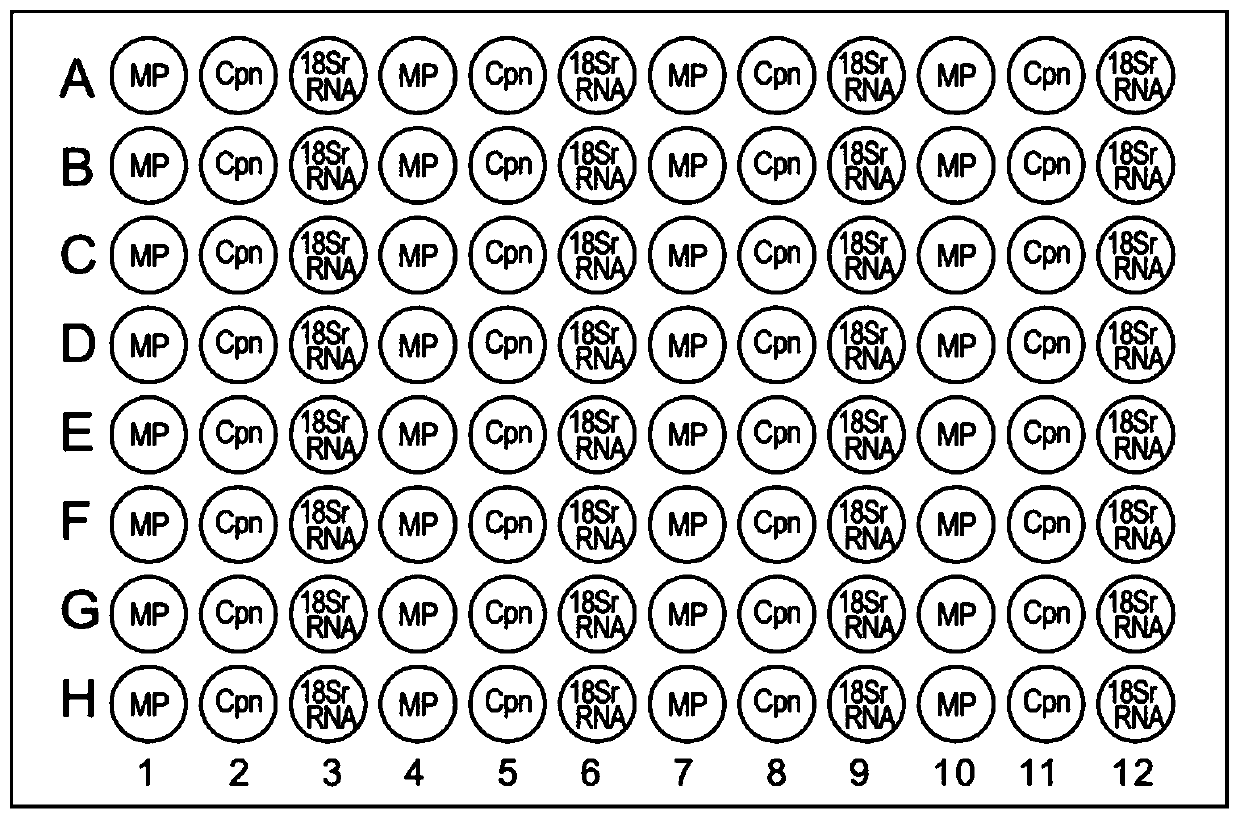
Mycoplasma pneumoniae and chlamydia pneumoniae nucleic acid combined detection kit and application thereof
A technology for Mycoplasma pneumoniae and Chlamydia pneumoniae, which is applied in the field of kits for the joint detection of Mycoplasma pneumoniae and Chlamydia pneumoniae nucleic acids based on double amplification technology, can solve problems such as easy pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] [Example 1] Sensitivity Test
[0085] The known concentrations of Mycoplasma pneumoniae and Chlamydia pneumoniae were diluted in a ten-fold concentration gradient. The pathogens at each dilution concentration were lysed with the lysate in the kit (pathogen: lysate = 1:1). Double amplification detects pathogens at each diluted concentration and assays the results. The pathogen dilutions of each gradient were separately repeated 5 times during the detection. The pathogen titer value with a positive detection rate of 90%-95% is initially used as the minimum detection limit. After the initial value of the detection limit is determined, the stock solution of each pathogen subtype is diluted to the vicinity of the initial value titer, and the pathogens of different concentrations are detected with this kit, and each titer is detected 20 times to further determine the minimum detection limit. Accurate determination (choose positive rate above 95%). The specific results are...
Embodiment 2
[0100] [Example 2] Specificity Verification
[0101] Different microorganisms were lysed with cell lysate and then double-amplified for detection to verify the specificity of the probe design of the kit. The results are shown in the table below:
[0102] Table 3 Cross-reaction verification data between other pathogenic microorganisms
[0103]
[0104] The results showed that the pathogens detected by this kit were not involved in cross-reaction with other pathogens.
Embodiment 3
[0105] [Example 3] Validation of clinical samples
[0106] 1. Clinical sample information
[0107] A total of 359 samples were tested in the Maternal and Child Health Hospital of Hubei Province, including 196 male samples and 163 female samples, accounting for 54.60% and 45.40% respectively. Among the 452 samples, the oldest patient was 62 years old, the youngest was 1 month old, the mean age was 10.63 years old, the standard deviation was 11.2 years old, and the median was 6 years old. The diagnosis results of the enrolled patients were all related to respiratory tract infection, and the specific distribution was as follows: 196 cases of suspected pneumonia, 69 cases of (acute, asthmatic) bronchitis, 37 cases of (acute) upper respiratory tract infection, 20 cases of (infectious) fever, 19 cases of influenza and 18 others.
[0108] 2. Test results
[0109] 1) Mycoplasma pneumoniae test results
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com