Preparation method of high-purity glimepiride
A high-purity, ethyl-based technology, applied in organic chemistry methods, organic chemistry and other directions, to achieve the effect of sufficient research on impurities, meeting the needs of use, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
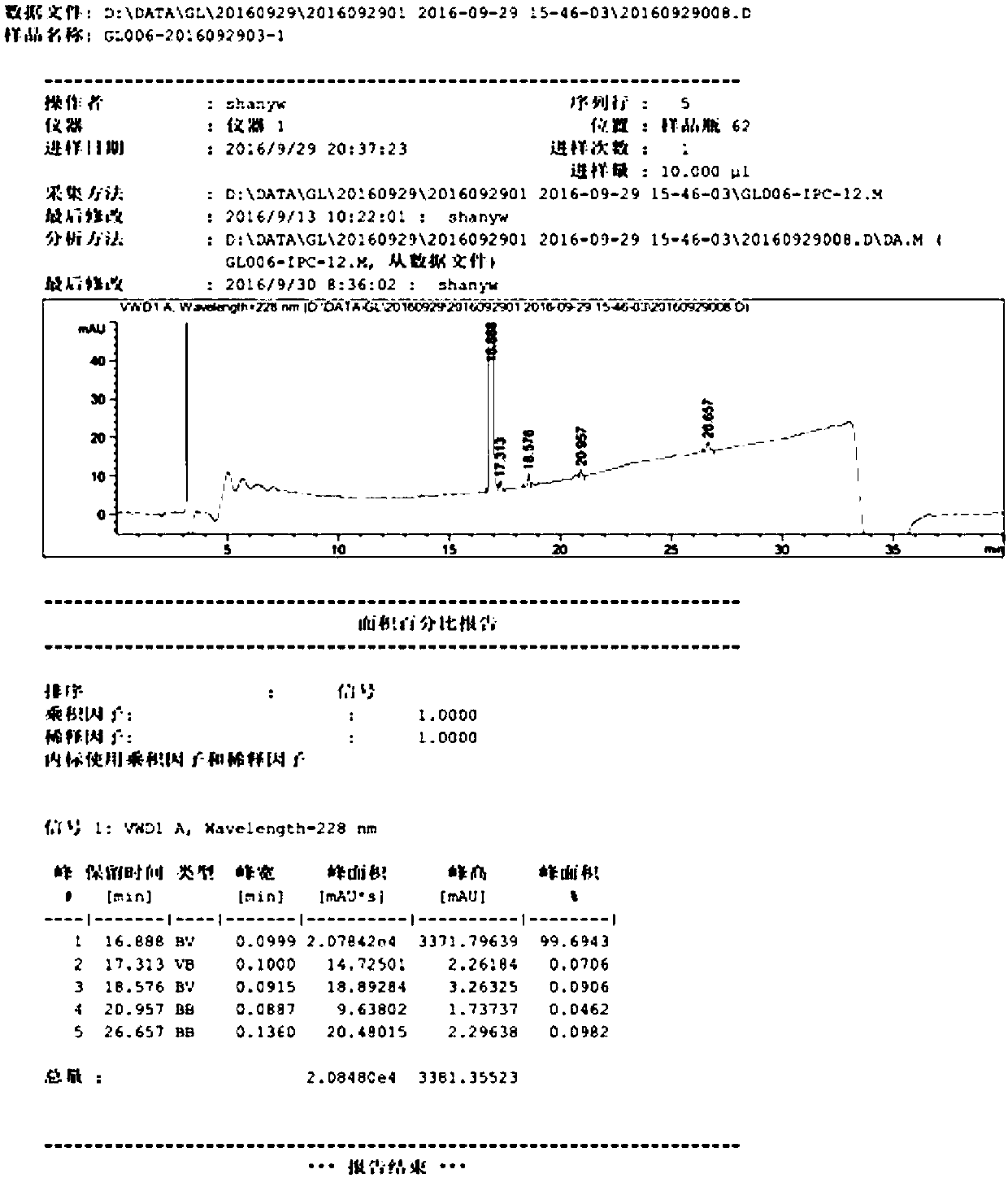
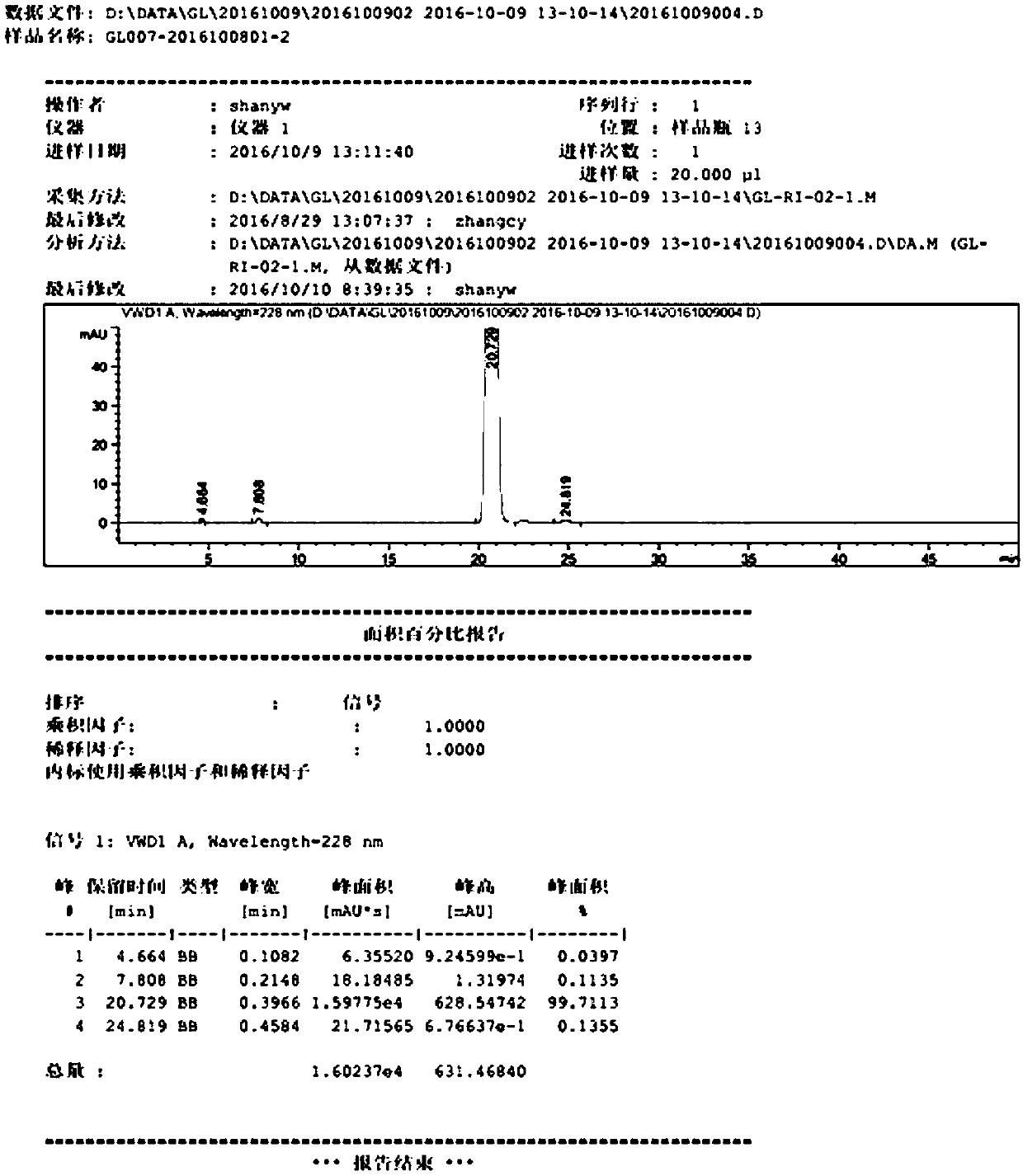
Image
Examples
Embodiment
[0030] A kind of preparation method of high-purity glimepiride, concrete steps are as follows:
[0031] Step 1, preparation of 3-ethyl-4-methyl-2-oxo-N-phenethyl-2,5-dihydro-1H-pyrrole-1-amide: pre-dehydration of toluene, molecular sieve preservation, the Toluene, 3-ethyl-4-methyl-3-pyrroline-2-one and 2-phenylethyl isocyanate were added to a three-necked flask, and the temperature was raised to reflux under nitrogen replacement, and the reaction was monitored by HPLC for about 4 hours. Slightly lower the temperature of the reaction product, and concentrate under reduced pressure at a water bath temperature of 50°C to remove toluene. After concentration to dryness, add acetone, stir at room temperature for 1 hour, cool down to about 0°C and crystallize for 2-3 hours, filter, and use as a filter cake. Rinse with a small amount of n-heptane and drain it, and dry it in a 45°C oven under reduced pressure to obtain 3-ethyl-4-methyl-2-oxo-N-phenylethyl-2,5-dihydro-1H -Pyrrole-1-ami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com