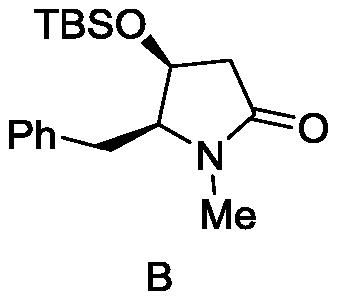
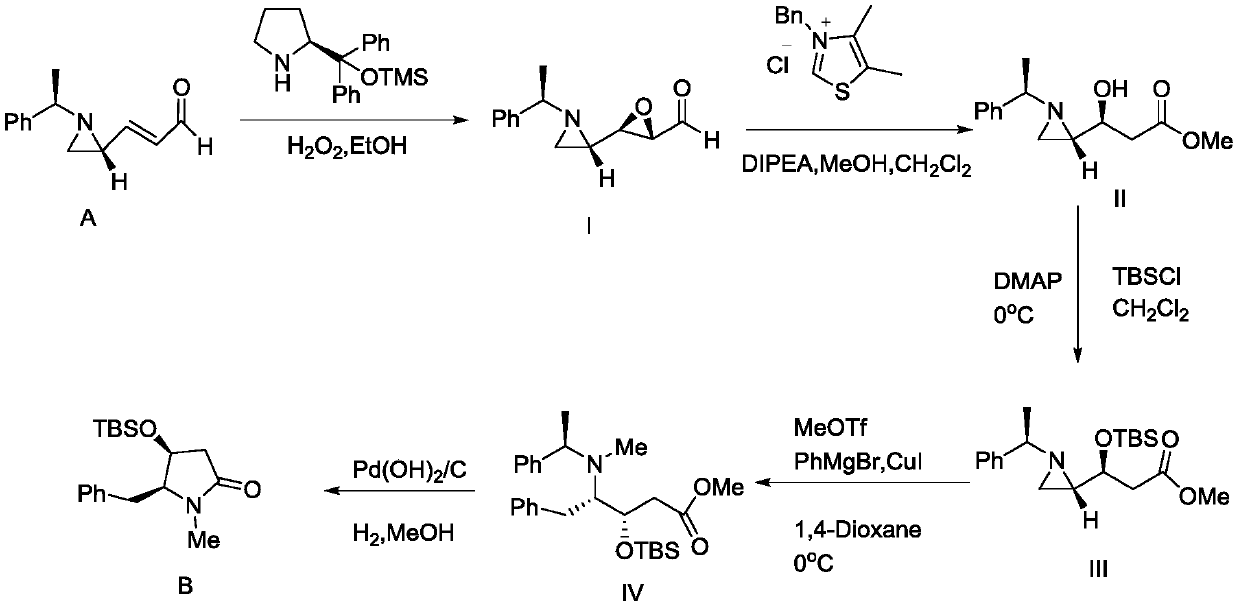
Preparation method of antitumor drug molecule (+)-Preussin intermediate
A technology for anti-tumor drugs and intermediates, which is applied in the field of preparation of anti-tumor drug molecules-Preussin intermediates, can solve the problems of limited reaction volume, easy configuration reversal, and difficult purification, and achieve good economy, simple steps, Solve the effect of many impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of intermediate compound described in formula (I)
[0036]
[0037] Dissolve the compound aziridine-unsaturated aldehyde (201mg) shown in formula (A) in ethanol (3.3mL), stir well, add catalyst (0.03mL, 0.01mol) and percentage concentration be 35% Hydrogen peroxide (0.2mL, 1.2mmol), after 5-7 hours of reaction, quenched with distilled water, then extracted three times with 10mL of dichloromethane, dried the collected organic phase with anhydrous sodium sulfate, vacuumed and removed the solvent The crude product was obtained, and 180 mg of the intermediate compound of formula (I) was obtained after separation by flash column chromatography, and the yield was 75%;
[0038] Wherein said catalyst chemical formula is:
[0039]
[0040] The coupling constant of the compound described in the formula (I) obtained wherein is: [α] D20=+57.1 (c1.00, CHCl 3 ); Rf = 0.35 (EtOAc / hexanes = 1:1); 1 H NMR (400MHz, CDCl 3 )δ8.92(d,J=6.2Hz,1H),7.38–7.21(m,5H),3.1...
Embodiment 2
[0043] The preparation of intermediate compound described in formula (II)
[0044]
[0045]Dissolve the compound (200mg, 0.92mmol) of the formula (I) prepared in Example 1 in dichloromethane (3ml), mix well, stir well at room temperature, then add NHC catalyst (0.023mL, 0.092mmol ), the above mixture was quenched with 5 mL of saturated ammonium chloride solution after 24 hours of reaction, and then extracted twice with 10 mL of ethyl acetate.
[0046] Then the collected organic phase was dried with anhydrous sodium sulfate, and the solvent was removed after vacuuming to obtain the crude product, which was separated by flash column chromatography to obtain 175mg of the intermediate compound described in formula (II), with a yield of 76%;
[0047] The coupling constant of the compound described in the formula (II) obtained wherein is: [α] D20=+35.6 (c1.00, CHCl3); Rf=0.28 (EtOAc / hexanes=1:1); 1H NMR (400MHz, CDCl3) δ7.42–7.26(m,5H),3.79(dt,J=7.7,4.8Hz,1H),3.63(s,3H),3.16(s,1...
Embodiment 3
[0050] The preparation of intermediate compound described in formula (III)
[0051]
[0052] Under the reaction condition of -5~5 ℃, in the reactor of nitrogen protection, add the intermediate compound (200mg, 0.92mmol) described in formula (II) prepared by the preparation method of Example 2 and dichloromethane (3mL), mix After uniformity, 200mg TBSCl (1.382mmol0 and DMAP (337mg, 2.763mmol) were added under magnetic stirring, and the above mixture was kept at -5-5°C. After stirring for 5-10 minutes, the temperature was slowly raised to room temperature. After reacting at room temperature for 6-8 hours, quench with saturated sodium bicarbonate solution (5mL), collect the organic phase, then extract the aqueous phase twice with dichloromethane solution (25mL), after the organic phases of collection are combined, use Wash with 10 mL of saturated brine, then dry with anhydrous sodium sulfate, filter, remove the solvent after vacuuming to obtain a crude product, and obtain 341 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com