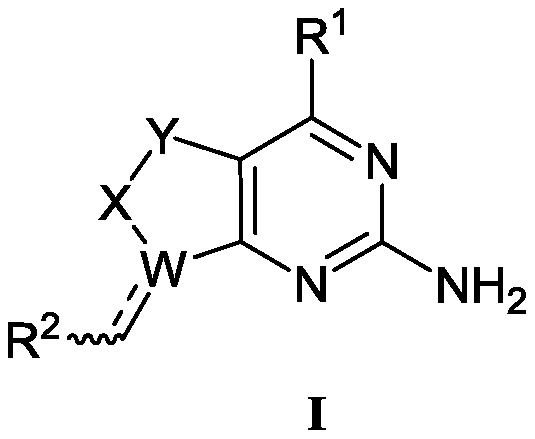
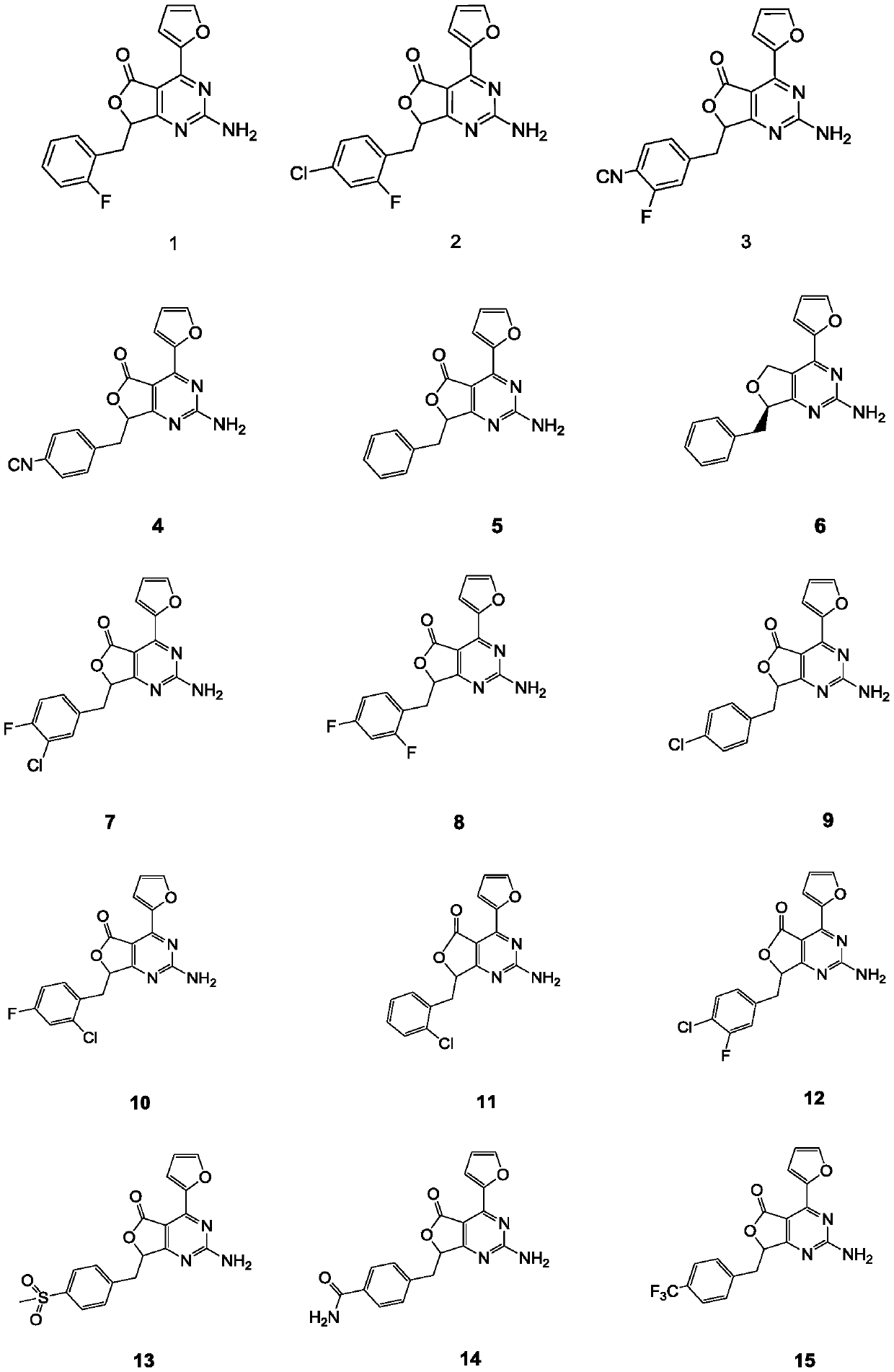
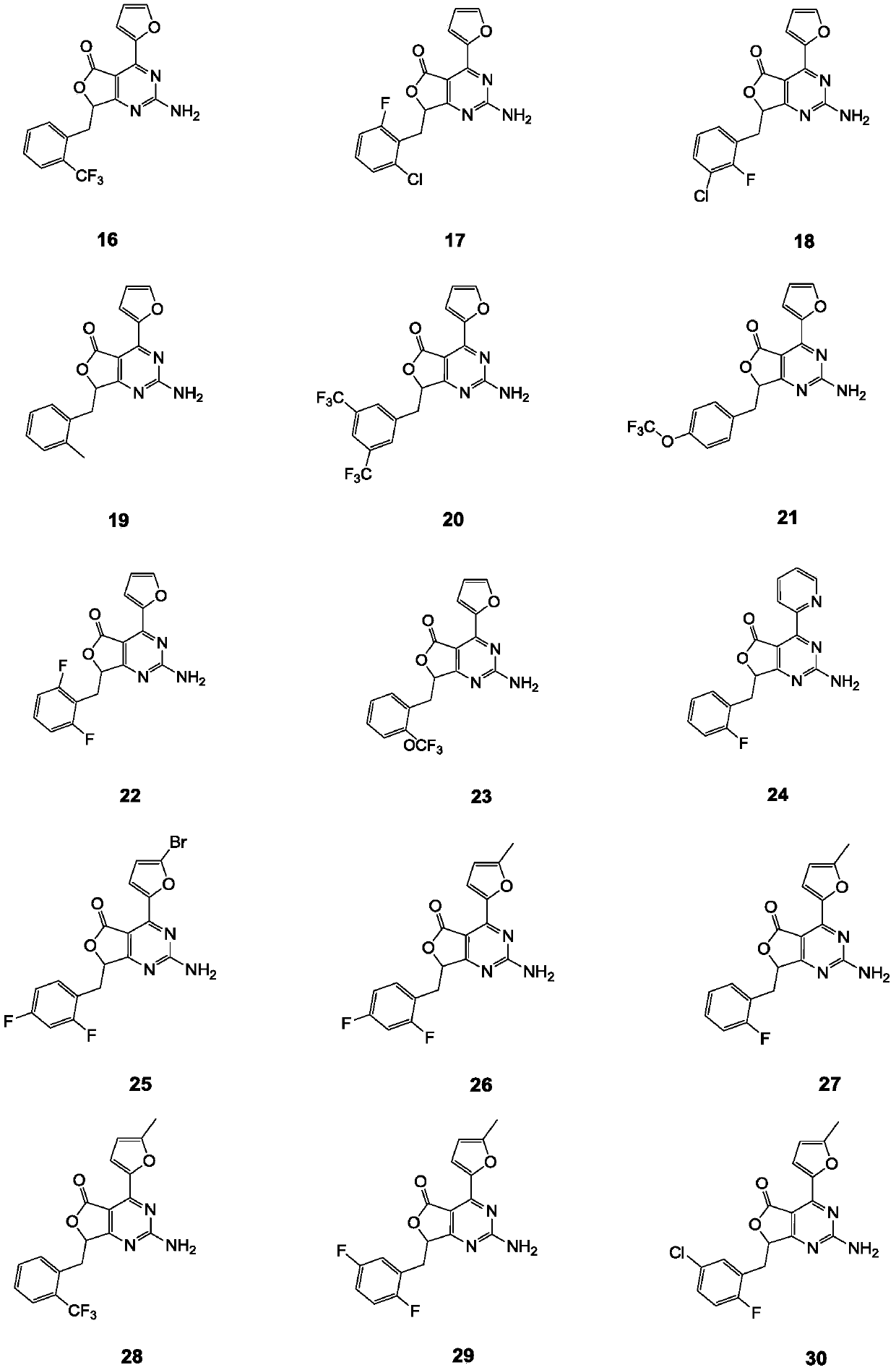
Aminopyrimidino-five-membered-heterocyclic compound, and intermediate, preparation method, medicine composition and application thereof
A compound and drug technology, applied in the field of aminopyrimido five-membered heterocyclic compounds, can solve problems such as tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Example 1: 2-amino-7-(2-fluorobenzyl)-4-(furan-2-yl)-5H,7H-furo[3,4-d]pyrimidin-5-one (compound 1)
[0166] synthetic route
[0167]
[0168] Synthesis of compound 1-c
[0169]Methyl 2,4-dichloro-6-methyl-pyrimidine-5-carboxylate (940mg, 4.25mmol), 2-furyltributylstannane (1.42g, 4.0mmol), tetrakis(triphenyl)phosphine palladium (260mg, 0.22mmol), and tetrahydrofuran (30mL) were stirred at 60°C under nitrogen for 16 hours. After cooling to room temperature, the reaction mixture was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / dichloromethane=3 / 1) to obtain white solid 1-c (870 mg, yield: 81%).
[0170] LC-MS(ESI):m / z=253[M+H] + .
[0171] Synthesis of compound 1-b
[0172] A mixture of compound 1-c (800 mg, 3.2 mmol), selenium dioxide (880 mg, 8.0 mmol) and dioxane (20 mL) was heated under reflux for 8 hours. After cooling to room temperature, the reaction mixture was concentrated under re...
Embodiment 2
[0179] Example 2: 2-amino-7-(4-chloro-2-fluorobenzyl)-4-(furan-2-yl)-5H,7H-furo[3,4-d]pyrimidin-5-one (compound 2)
[0180] synthetic route
[0181]
[0182] Synthesis of compound 2-f
[0183] Dissolve furfural (9.6 g, 0.1 mol), O-methylisourea sulfate (20.64 g, 0.12 mol) and ethyl acetoacetate (14.3 g, 0.11 mol) in anhydrous N,N-dimethylformamide (200 mL), sodium bicarbonate (33.6 g, 0.4 mmol) was added to the solution. The reaction mixture was heated to 70°C under nitrogen protection, stirred for 3 hours and then cooled to room temperature. Add saturated brine (300mL), a large amount of yellow suspension appeared, extracted with ethyl acetate (500mL×2), combined the organic phases, washed with water (200mL) and saturated brine (100mL), dried over anhydrous sodium sulfate, and reduced pressure After concentration, the residue was purified by silica gel column chromatography (petroleum ether:ethyl acetate=6-3:1) to obtain light yellow solid 2-f (15.0 g, yield: 57%). LC...
Embodiment 3
[0200] Example 3: 4-{[2-amino-4-(furan-2-yl)-5-oxo-5H,7H-furo[3,4-d]pyrimidin-7-yl]methyl-2 -Fluorobenzonitrile (Compound 3)
[0201]
[0202] Using 2-d and 2-fluoro-4-cyanobenzyl bromide as raw materials, the synthesis method is the same as in Example 2.
[0203] LC-MS(ESI):m / z=351[M+H] + .
[0204] 1 H NMR (500MHz, d 6 -DMSO) δ: 8.27(d, J=3.5Hz, 1H), 8.11(brs, 1H), 8.03(s, 1H), 7.92(brs, 1H), 7.89(t, J=7.5Hz, 1H), 7.50(d, J=10.0Hz, 1H), 7.34(d, J=8.0Hz, 1H), 6.78(dd, J=1.5Hz, 3.5Hz, 1H), 5.67(dd, J=3.5Hz, 8.5Hz , 1H), 3.45 (dd, J = 3.5Hz, 15.0Hz, 1H), 3.14 (dd, J = 8.5Hz, 15.0Hz, 1H) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com