Erannis ankeraria Staudinger sex pheromone composition, synthetic method and identification of (Z,Z,Z)-3,6,9-nonadecatriene, and intelligent application system of composition
A synthetic method, larch technology, applied in the direction of botanical equipment and methods, hydrocarbons, hydrocarbons, etc., can solve problems such as waste, and achieve the effects of improving delivery efficiency, saving costs, and high reliability of results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Propylene oxide derivatives [(6Z,9Z)-cis-3,4-epoxynonadecadiene: (3Z,9Z)-cis-6,7-epoxynonadecadiene: Preparation of (3Z,6Z)-cis-9,10-epoxy nonadecadiene]:
[0048] The propylene oxide derivative in the present invention can be synthesized by the route described below.
[0049] A solution of (3Z,6Z,9Z)-nonadecatriene (2.62g, 10mmol) in 10mL of dichloromethane was slowly added dropwise to a dichloromethane solution of m-chloroperoxybenzoic acid (2.06g, 12mmol) under stirring at room temperature. In methane solution, stirred for reaction 1h, filtered off insolubles, washed with saturated sodium bicarbonate solution, evaporated to remove solvent, separated by column chromatography, petroleum ether / diethyl ether (30:1), obtained epoxy isomer mixture (2.08 g, 75%). GC analysis showed that (6Z,9Z)-cis-3,4-epoxynonadecadiene: (3Z,9Z)-cis-6,7-epoxynonadecadiene: (3Z,6Z)-cis -9,10-epoxynonadecadiene=1:1:1.
Embodiment 2
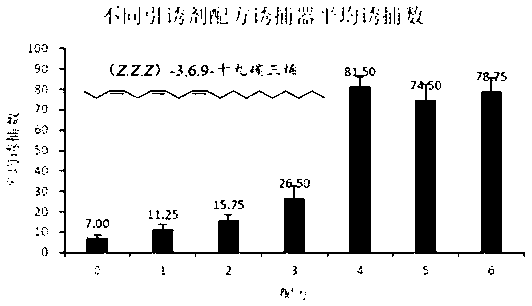
[0050] Embodiment 2: Different attractant formulations lure Larch inchworm lure monitoring effect experiment
[0051] Experimental locations: Saihanba Machinery Forest Farm in Hebei and Sumushan Forest Farm in Hohhot, Inner Mongolia.
[0052] Experimental method: Prepare the formula in Table 1 into lure cores and place them in triangular traps. The traps are spaced about 20m apart and 1.5m above the ground. Random block design is adopted. Each treatment is repeated 4 times, and the investigation is continued for 10 days, every two days Count the number of insects trapped in each trap, and remove the adults trapped in the trap.
[0053] Table 1 Lure recipe
[0054]
[0055] Experimental results: see the lure effect figure 1 .
[0056] Depend on figure 1 It can be seen that the combination of (3Z,6Z,9Z)-nonadecatriene: propylene oxide derivative [(6Z,9Z)-cis-3,4-epoxynonadecadiene: (3Z,9Z)-cis -6,7-epoxynonadecadiene:(3Z,6Z)-cis-9,10-epoxynonadecadiene=1:1:1] When the ma...
Embodiment 3
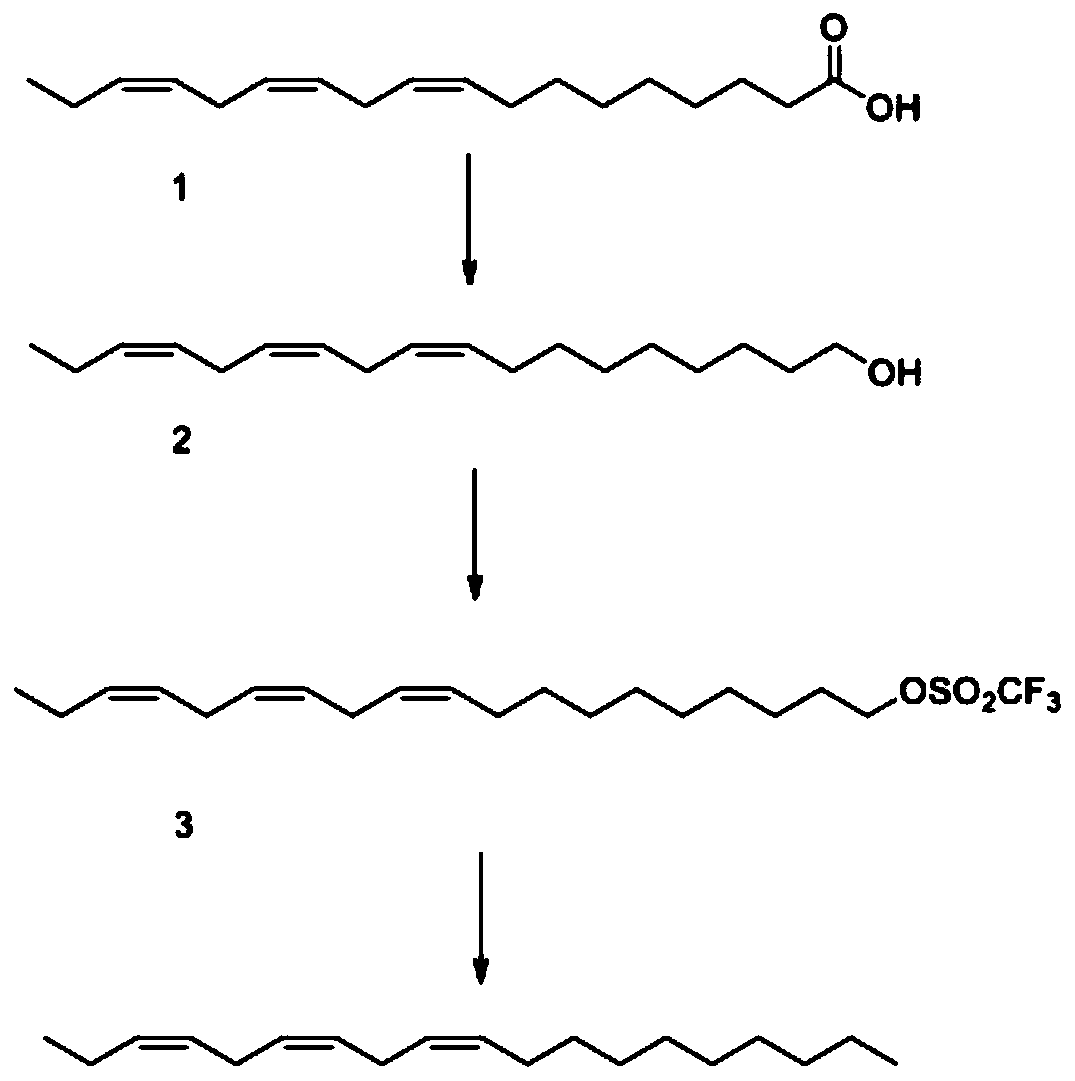
[0058] In the present invention, the synthetic route of the main active ingredient of the larch inchworm sex pheromone is as follows: figure 2 shown.
[0059] Step 1: Under the action of sodium triacetoxyborohydride, α-linolenic acid (the structural formula is shown as compound 1 in formula a) is subjected to a reduction reaction at 20°C for 3 hours, and the α-linolenic acid and triacetoxyborohydride are The molar ratio of sodium is preferably 1:1, and the solvent for the reduction reaction is tetrahydrofuran. Obtain (Z, Z, Z)-9,12,15-octadecatrien-1-alcohol (structural formula such as figure 2 indicated by the topmost first arrow).
[0060] Wherein, the reducing agent is dissolved in the solvent, and then alpha-linolenic acid is slowly added into the solution for reaction, and the reaction time is calculated from the time when the alpha-linolenic acid is added. After the reaction was terminated, it was extracted with n-hexane to obtain an organic phase, which was dried o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com