Synthesis method of 2-(5-aryl-1,3,4-oxadiazol-2-yl)aniline compound
A technology of aniline compounds and synthesis methods, which is applied in the direction of electrolysis process, electrolysis components, electrolysis organic production, etc., can solve the problems of lack of application value, long reaction time, low efficiency, etc., and achieve the green environmental protection of the preparation process and short reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] A kind of synthetic method of 2-(5-aryl-1,3,4-oxadiazol-2-yl) aniline compound, comprises the steps:
[0063] Electrocatalytic reaction, electrolyte, isatin compound, benzohydrazide compound, solvent, alkali, respectively, are added to the reaction cell, and catalytic electrodes are installed, and the reaction is stirred with electricity;
[0064] Separation and purification, separation and purification of the solution after the electrocatalytic reaction is completed to obtain 2-(5-aryl-1,3,4-oxadiazol-2-yl)aniline compounds; the 2-(5-aryl- 1,3,4-oxadiazol-2-yl)aniline compounds have the structure shown below:
[0065]
[0066] Among them, R 1 for hydrogen, C 1 ~C 5 Alkyl, C 1 ~C 5 One or more of alkoxy and halogen;
[0067] R 2 is phenyl, substituted phenyl, heterocyclyl, and the substituents in the substituted phenyl are trifluoromethyl, halogen, C 1 ~C 5 Alkyl and C 1 ~C 5 of alkoxy.
[0068] Specifically, in a 10mL non-separated electrolytic cell, the...
Embodiment 1
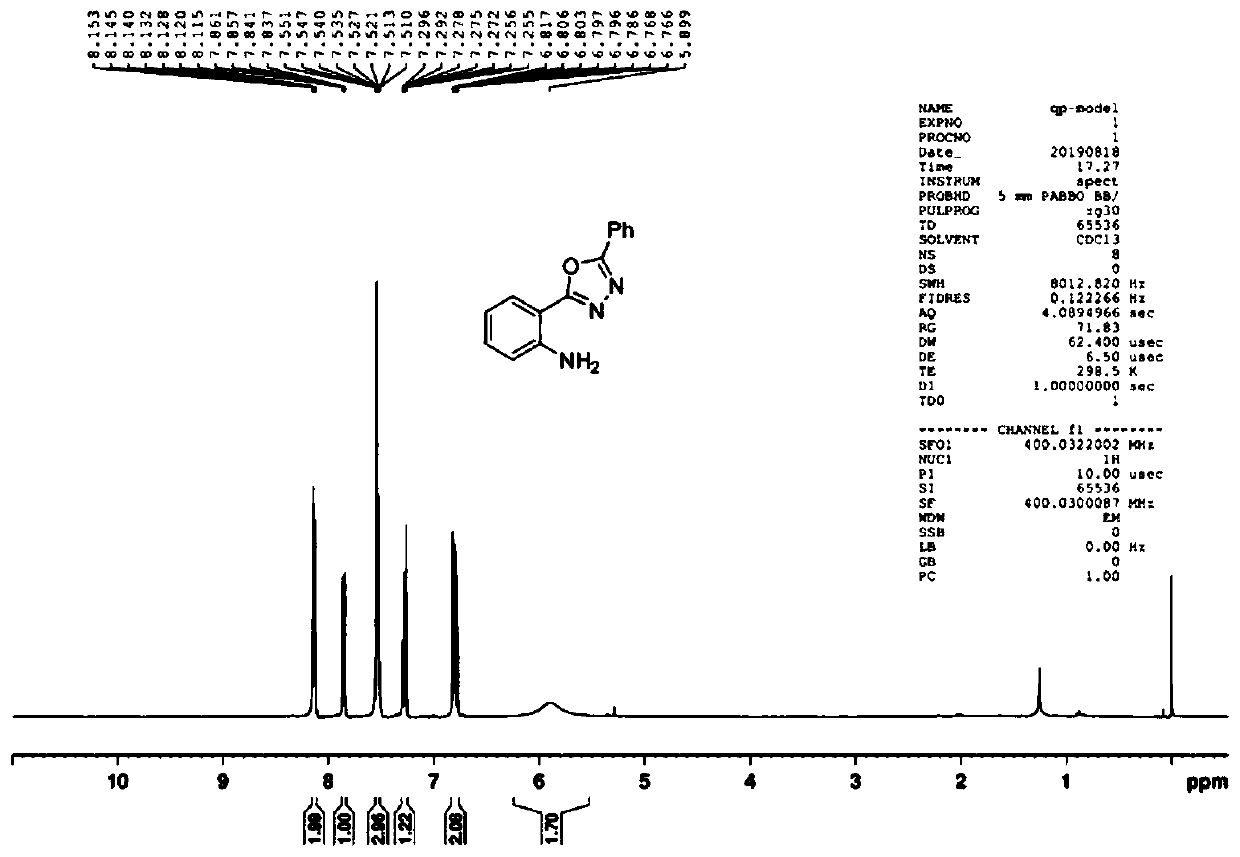
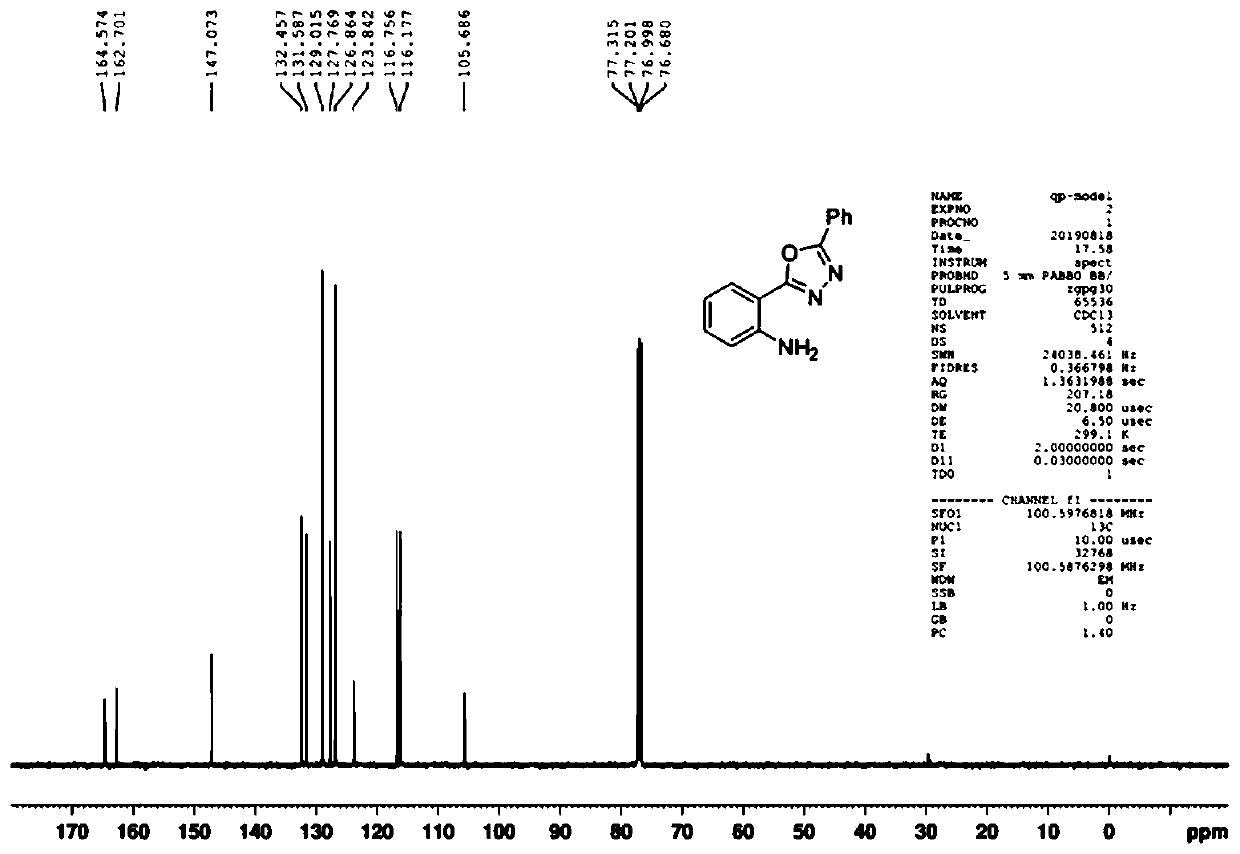
[0074] Example 1: Put isatin (0.3mmol, 44.1mg), benzohydrazide (0.3mmol, 40.8mg), potassium iodide (0.3mmol, 49.8mg), potassium carbonate (0.3mmol , 41.5mg) and dimethyl sulfoxide (3.0mL), the platinum plate electrode was used as both anode and cathode, and reacted under electric stirring (I=10mA) at 120°C. After the reaction was completed (TLC tracking detection), the residue obtained by spinning was used as the eluent to pass the chromatographic column to obtain the product 2-(5-phenyl-1,3,4-oxadiazole- 2-yl) aniline compound with a yield of 77%.
[0075] The 2-(5-phenyl-1,3,4-oxadiazol-2-yl) aniline product is analyzed by nuclear magnetic resonance spectrometer, the results can be found in Figure 1~2 , figure 1 For the 2-(5-phenyl-1,3,4-oxadiazol-2-yl) aniline product that the embodiment of the present invention 1 provides 1 H NMR ( 1 H-NMR) spectrogram; figure 2 For the 2-(5-phenyl-1,3,4-oxadiazol-2-yl)aniline product provided in Example 1 of the present invention ...
Embodiment 2
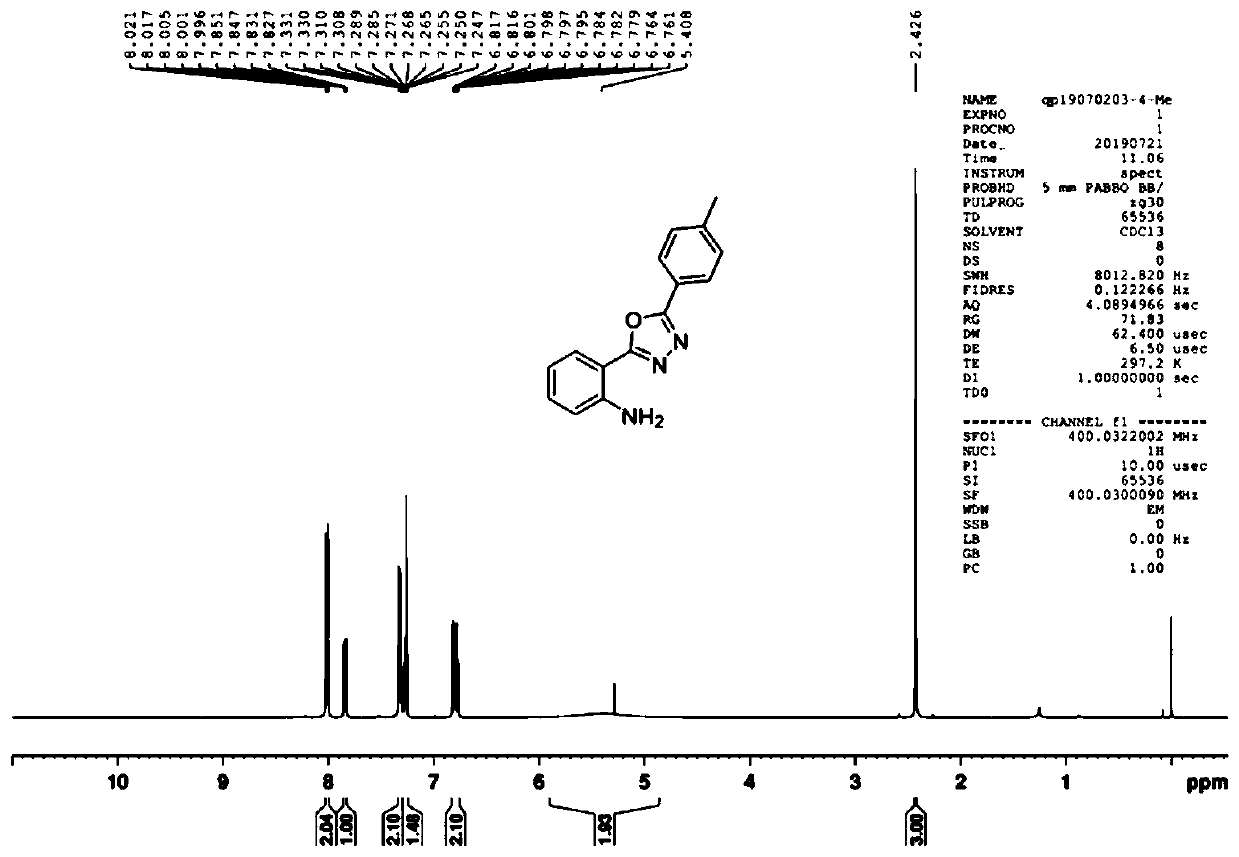
[0079] Example 2: Put isatin (0.3mmol, 44.1mg), 4-methylbenzohydrazide (0.3mmol, 45.1mg), potassium iodide (0.3mmol, 49.8mg), carbonic acid in a 10mL electrolytic cell without separation Potassium (0.3 mmol, 41.5 mg) and dimethyl sulfoxide (3.0 mL) were reacted at 120° C. under electric stirring (I=10 mA), and the platinum plate electrode was used as both the anode and the cathode. After the reaction was completed (TLC tracking detection), the residue obtained by spinning was used as an eluent to pass the chromatographic column to obtain the product 2-(5-(p-methylphenyl)-1,3, 4-oxadiazol-2-yl)aniline compound, the yield was 83%.
[0080] The 2-(5-(p-methylphenyl)-1,3,4-oxadiazol-2-yl) aniline product is analyzed by nuclear magnetic resonance spectrometer, the results can be found in Figure 3-4 , image 3 For the 2-(5-(p-methylphenyl)-1,3,4-oxadiazol-2-yl)aniline product that the embodiment of the present invention 2 provides 1 H NMR ( 1 H-NMR) spectrogram; Figure 4 For...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com