Core-shell nickel ferrite and its preparation method, nickel ferrite@c material and its preparation method and application
A nickel ferrite, core-shell technology, applied in chemical instruments and methods, carbon preparation/purification, nanotechnology for materials and surface science, etc., can solve the problems of serious material agglomeration and poor cycle performance, and achieve product Good performance, low cost, and the effect of preventing electrochemical wear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] (1) First, add 8 mL of glycerin to 40 mL of isopropanol and stir evenly, then add 0.0363 g of Ni (NO 3 ) 2 ·6H 2 O and 0.101g of Fe(NO 3 ) 3 9H 2 O, stir well at room temperature.
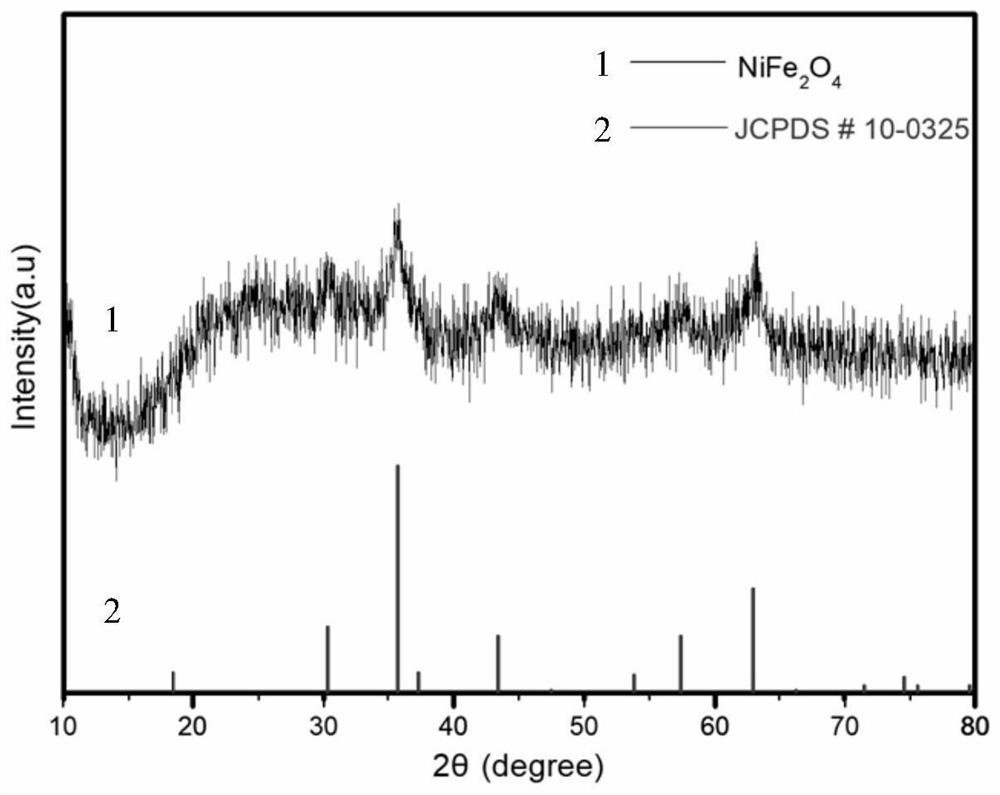
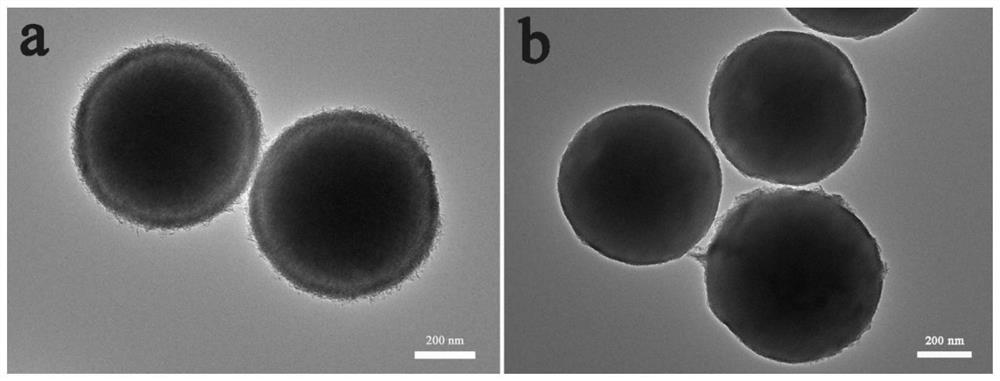
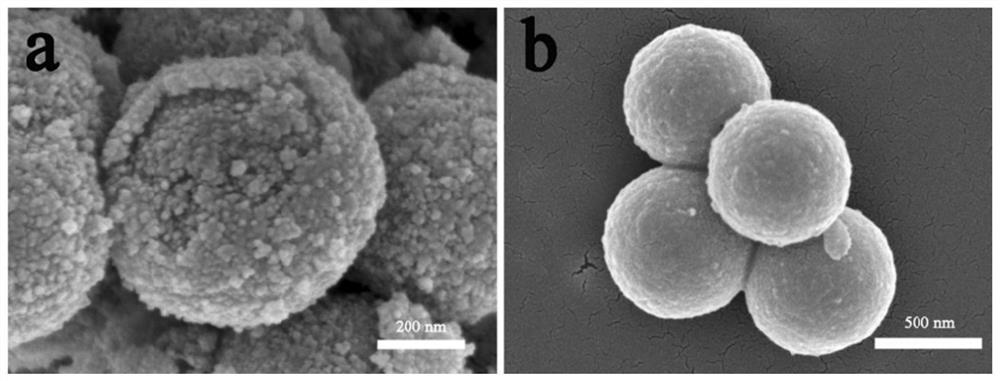
[0063] (2) Transfer the homogeneous liquid obtained in step (1) to a 100mL polytetrafluoroethylene-lined autoclave. After solvothermal reaction at 180°C for 6 hours, cool to room temperature naturally, and then centrifuge, wash, and dry. Obtain yellow nickel-iron glyceride ball powder; in the air, heat up to 400°C at 1°C / min for 2 hours, then cool naturally to room temperature to obtain core-shell NiFe 2 o 4 .
[0064] (3) the core-shell NiFe obtained in step (2) 2 o 4 Ultrasonic dispersion in the mixed solution of 10mL water and 20mL ethanol, add 1mLNH 3 ·H 2 O (28wt%), 1g resorcinol and 0.12mL formaldehyde, stirred for 2h. Core-shell NiFe was obtained by centrifugation, washing and drying 2 o 4 @RF composite material, under an argon atmosphere, heat up to 600 ° C for 2 h to o...
Embodiment 2
[0066] (1) First, add 8 mL of glycerin to 40 mL of isopropanol and stir evenly, then add 0.0363 g of Ni (NO 3 ) 2 ·6H 2 O and 0.101g of Fe(NO 3 ) 3 9H 2 O, stir well at room temperature.
[0067] (2) Transfer the homogeneous liquid obtained in step (1) to a 100mL polytetrafluoroethylene-lined autoclave. After solvothermal reaction at 180°C for 6 hours, cool to room temperature naturally, and then centrifuge, wash, and dry. Obtain yellow nickel-iron glyceride spherical powder; in air, heat up to 400°C at 2°C / min for calcination for 2h, then cool naturally to room temperature to obtain solid spherical NiFe 2 o 4 .
[0068] (3) the solid spherical NiFe that step (2) obtains 2 o 4 Ultrasonic dispersion in the mixed solution of 10mL water and 20mL ethanol, add 1mLNH 3 ·H 2 O (28wt%), 1g resorcinol and 0.12mL formaldehyde, stirred for 2h. Core-shell NiFe was obtained by centrifugation, washing and drying 2 o 4 @RF composite material, under an argon atmosphere, heat up ...
Embodiment 3
[0070] (1) First, add 8 mL of glycerin to 40 mL of isopropanol and stir evenly, then add 0.0363 g of Ni (NO 3 ) 2 ·6H 2 O and 0.101g of Fe(NO 3 ) 3 9H 2 O, stir well at room temperature.
[0071] (2) Transfer the homogeneous liquid obtained in step (1) to a 100mL polytetrafluoroethylene-lined autoclave. After solvothermal reaction at 160°C for 8 hours, cool to room temperature naturally, and then centrifuge, wash, and dry. Obtain yellow nickel-iron glyceride ball powder; in the air, heat up to 400°C at 1°C / min for 2 hours, then cool naturally to room temperature to obtain core-shell NiFe 2 o 4 .
[0072] (3) the solid spherical NiFe that step (2) obtains 2 o 4 Ultrasonic dispersion in the mixed solution of 10mL water and 20mL ethanol, add 1mLNH 3 ·H 2 O (28wt%), 1g resorcinol and 0.12ml formaldehyde, stirred for 2h. Core-shell NiFe was obtained by centrifugation, washing and drying 2 o 4 @RF composite material, in an argon atmosphere, under an inert atmosphere, h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com