Compound bitter gourd peptide oral medicine for activating insulin and treating diabetes, and preparation method of compound bitter gourd peptide oral medicine
A technology of balsam pear peptide and insulin, which is applied to the preparation methods of peptides, chemical instruments and methods, pharmaceutical formulations, etc., can solve problems such as injury, normal islet function, injury and side effects, and achieve the effect of improving production efficiency and purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
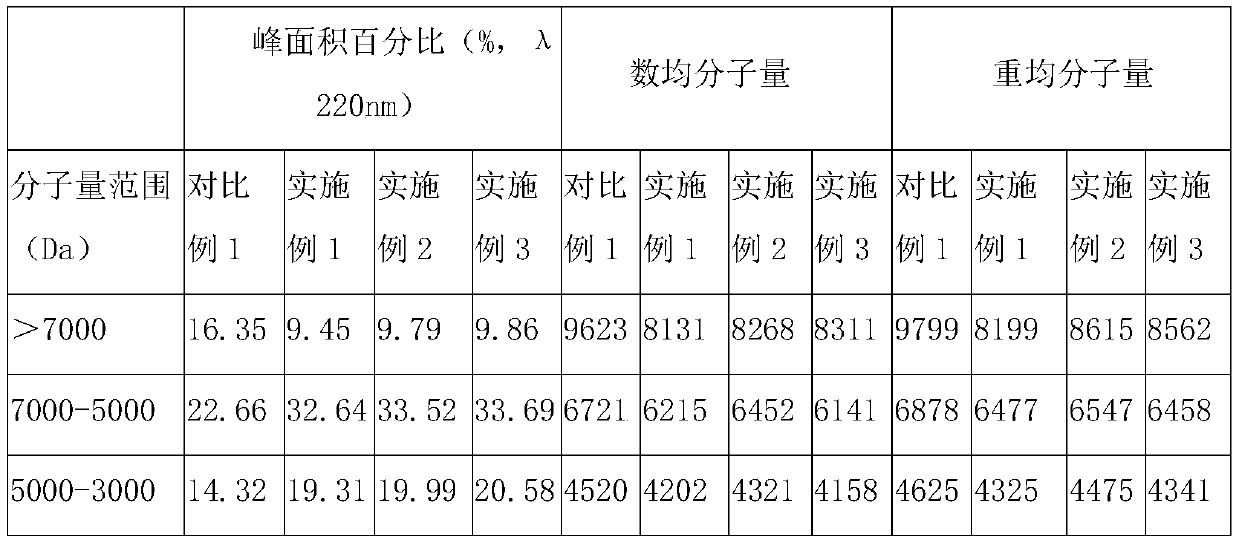
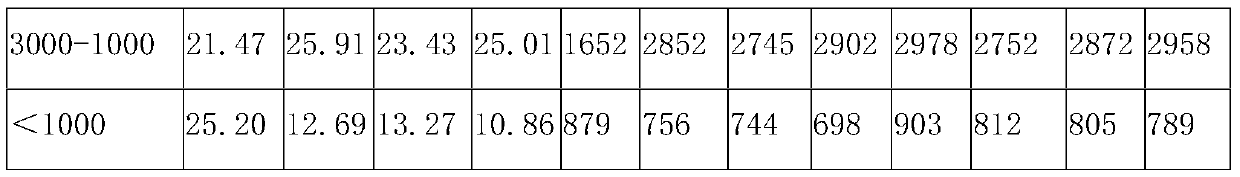
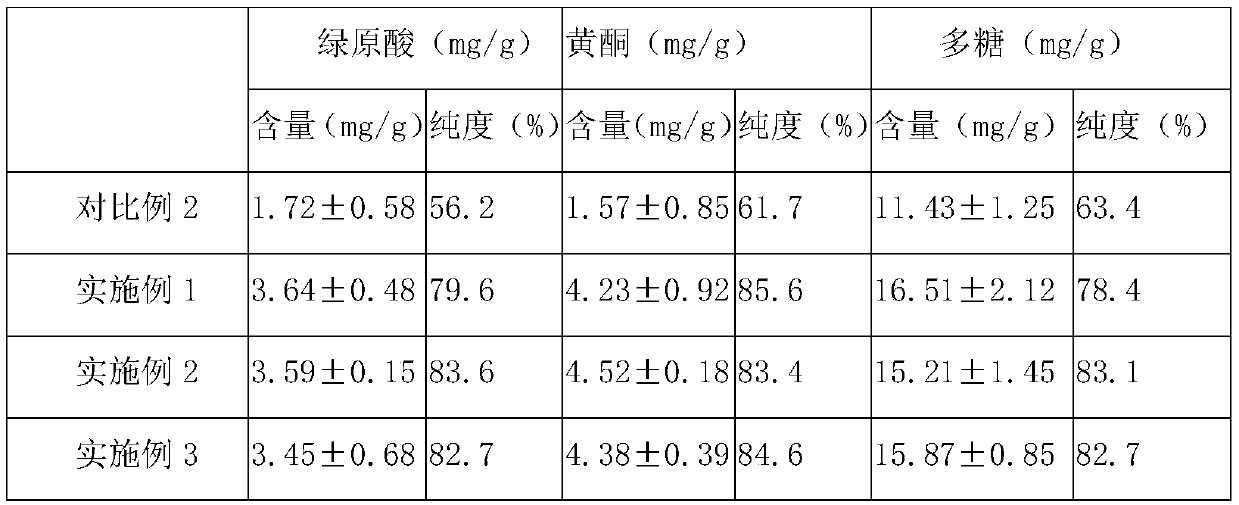
[0053] In parts by weight, the compound bitter melon peptide oral medicine for activating insulin and treating diabetes in this embodiment includes: 20 parts of bitter melon peptide powder, 4 parts of American ginseng, 10 parts of astragalus, 3 parts of ganoderma lucidum powder, 8 parts of yam powder, and 10 parts of wheat bran. 10 parts of guava leaf powder, 5 parts of onion extract, 5 parts of wolfberry, 12 parts of chrysanthemum notoginseng extract, 1 part of coix seed, 5 parts of konjac glucomannan, 8 parts of lotus leaf, oligomeric wood 5 parts sugar.
[0054] Further, the preparation method of the bitter melon peptide comprises the following steps:
[0055] S11. Take one or more of fresh balsam pear, dried balsam pear, and balsam pear seeds as balsam pear raw material, add deionized water 5 times the weight of balsam pear raw material, and soak at 25°C for 10-12h (preferably 10.5h), take out Then rinse with deionized water 2-3 times to remove pesticide residues and impu...
Embodiment 2
[0088] The only difference between this example and Example 1 is that, in parts by weight, the compound bitter melon peptide oral medicine for activating insulin and treating diabetes in this example consists of the following components: 30 parts of bitter melon peptide powder, 6 parts of American ginseng , 12 parts of astragalus, 5 parts of Ganoderma lucidum powder, 10 parts of yam powder, 15 parts of wheat bran, 12 parts of guava leaf powder, 10 parts of onion extract, 10 parts of wolfberry, 15 parts of chrysanthemum notoginseng extract, 2 coix seed 8 parts, 8 parts of konjac glucomannan, 10 parts of lotus leaf, 8 parts of xylooligosaccharide.
[0089]
[0090] The only difference between this example and Example 1 is that, in parts by weight, the compound bitter melon peptide oral medicine for activating insulin and treating diabetes in this example consists of the following components: 25 parts of bitter melon peptide powder, 5 parts of American ginseng , 11 parts of ast...
Embodiment 4
[0103] This embodiment also provides a preparation method of compound bitter gourd peptide oral medicine for activating insulin and treating diabetes, which includes:
[0104] S100. Prepare the bitter melon peptide powder and the chrysanthemum notoginseng extract respectively according to the preparation method of the balsam pear peptide powder and the chrysanthemum notoginseng extract described in one of embodiments 1-3;
[0105] S200. Weigh each component according to the dosage of the components in the compound bitter gourd peptide oral medicine described in one of the embodiments 1-3;
[0106]S300. Soak the American ginseng, astragalus, ganoderma powder, yam powder, wheat bran, guava leaf powder, onion extract, coix seed, lotus leaf, and wolfberry in water for 5-8 hours (preferably 6 hours), and use The weight of the soaked water is 5-8 times (preferably 7 times) of the total weight of American ginseng, astragalus, ganoderma powder, yam powder, wheat bran, guava leaf powde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com