Method for electrochemical synthesis of lithium imidodifluorosulfonate
A technology of lithium bisfluorosulfonate imide and bisfluorosulfonate imide is applied in the field of electrochemical synthesis of lithium bisfluorosulfonate imide, which can solve the problem that the purity of bisfluorosulfonate imide is difficult to meet the battery-grade standard , can not directly enter the large-scale industrial application and other problems, to achieve the effect of low cost, low energy consumption and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A method for electrochemically synthesizing lithium bisfluorosulfonate, comprising the following steps:
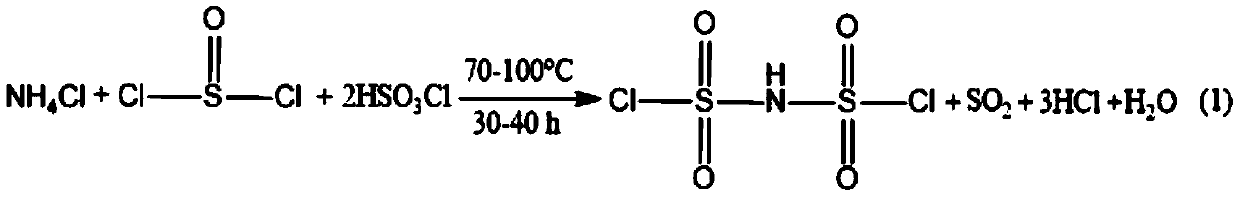
[0031] Step 1, synthesis of dichlorosulfonate imide: under magnetic stirring (1000-1500 rpm), add 300g thionyl chloride (SOCl 2 ) and 500g chlorosulfonic acid (CISO 3 H) Add 110g of ammonium chloride (NH 4 Cl), heating the temperature of the mixed solution to 100°C to react and generate SO 2 And HCl gas, brought out with nitrogen, absorbed with sodium hydroxide (1M NaOH) aqueous solution; distilled after 30 hours of reaction, collected fractions at 115-120°C, and cooled to obtain 185g of colorless dichlorosulfonic acid imide (HCSI) liquid , the yield of dichlorosulfonic acid imide (HCSI) is 89.6%;
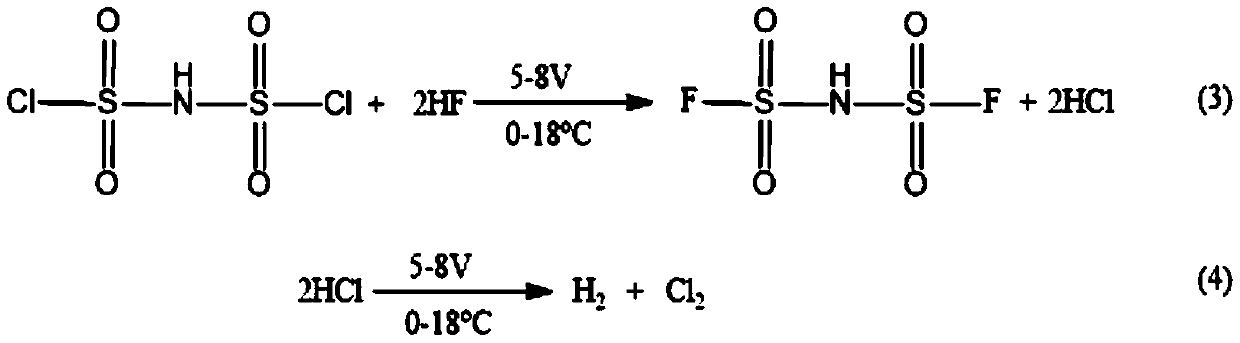
[0032] Step 2, reduce the water content of anhydrous hydrofluoric acid: the temperature of polytetrafluoroethylene electrolytic cell is adjusted to 18 ℃, with two nickel plates respectively as anode and negative electrode, add 300ml anhydrous hydrofluoric acid ( HF),...
Embodiment 2
[0040] A method for electrochemically synthesizing lithium bisfluorosulfonate, comprising the following steps:
[0041] Step 1, synthesis of dichlorosulfonic acid imide: under magnetic stirring (1000-1500 rev / min), add 350g thionyl chloride (SOCl 2 ) and 450g chlorosulfonic acid (CISO 3 H) Add 100g ammonium chloride (NH 4 Cl), heating to raise the temperature of the mixed solution to 100°C to react, generate SO2 and HCl gas, take it out with nitrogen, and absorb it with sodium hydroxide (1M NaOH) aqueous solution; distill after 40 hours of reaction, collect 115-120°C Fraction, after cooling, obtain 200g colorless dichlorosulfonic acid imide (HClSI) liquid, and the yield of dichlorosulfonic acid imide (HClSI) is 90.6%;
[0042] Step 2, reduce the water content of anhydrous hydrofluoric acid: the temperature of polytetrafluoroethylene electrolytic cell is adjusted to 18 ℃, with two nickel plates respectively as anode and negative electrode, add 300ml anhydrous hydrofluoric aci...
Embodiment 3
[0049] A method for electrochemically synthesizing lithium bisfluorosulfonate, comprising the following steps:
[0050] Step 1, synthesis of dichlorosulfonic acid imide: under magnetic stirring (1000-1500 rpm), add 320g thionyl chloride (SOCl 2 ) and 480g chlorosulfonic acid (CISO 3 H) Add 105g ammonium chloride (NH 4 Cl), heating the temperature of the mixed solution to 80°C to react and generate SO 2 And HCl gas, carried out with nitrogen, absorbed with sodium hydroxide (NaOH) aqueous solution; distilled after 35 hours of reaction, collected fractions at 115-120 ° C, and obtained 190 g of colorless dichlorosulfonic acid imide (HClSI) liquid after cooling, The yield of dichlorosulfonic acid imide (HClSI) is 91.5%;
[0051] Step 2, reduce the water content of anhydrous hydrofluoric acid: the temperature of polytetrafluoroethylene electrolytic cell is adjusted to 18 ℃, with two nickel plates respectively as anode and negative electrode, add 300ml anhydrous hydrofluoric acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com