Synthesis method of 4-(difluoromethyl)-2-hydroxypyridine-5-sulfonyl chloride
A synthesis method and hydroxypyridine technology, applied in the direction of organic chemistry and the like, achieve the effects of easy realization, mild reaction conditions, and simple synthesis operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
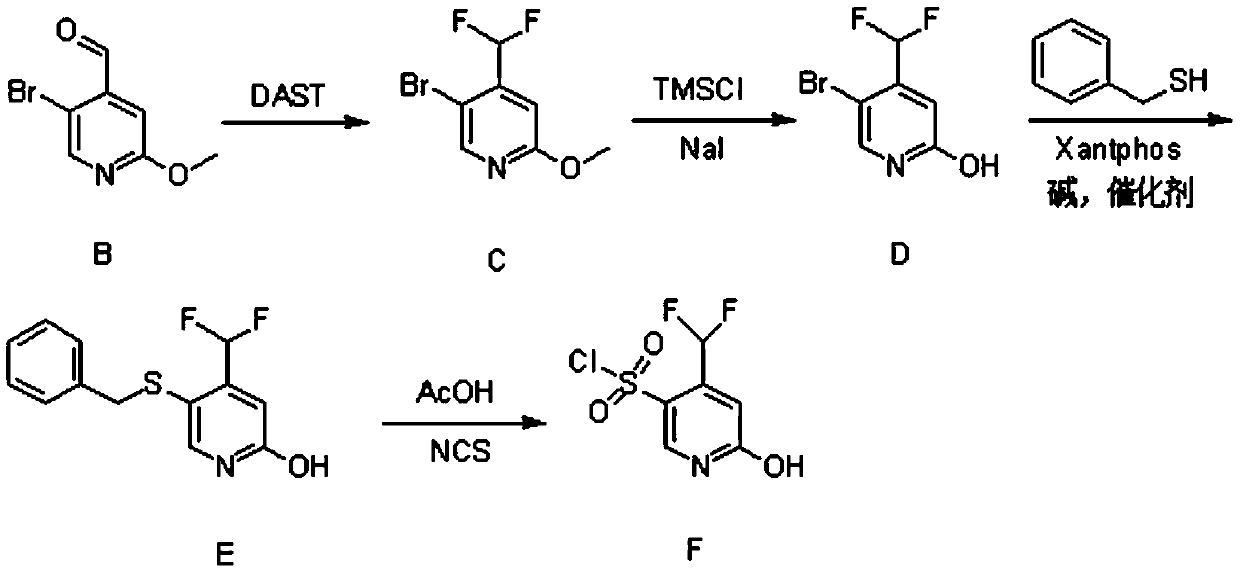
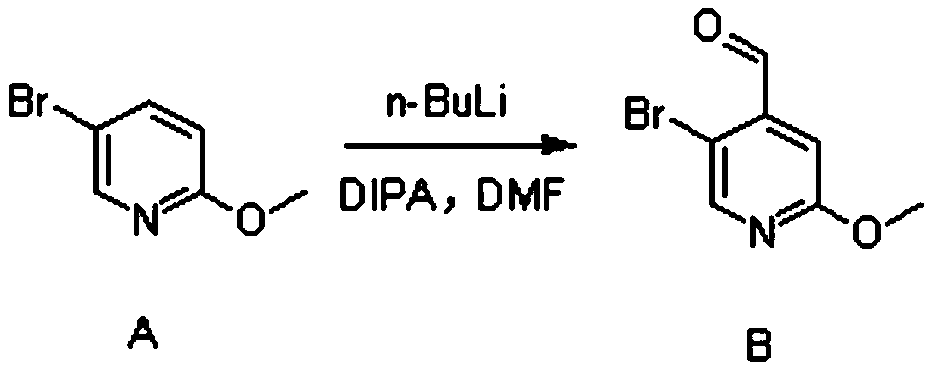
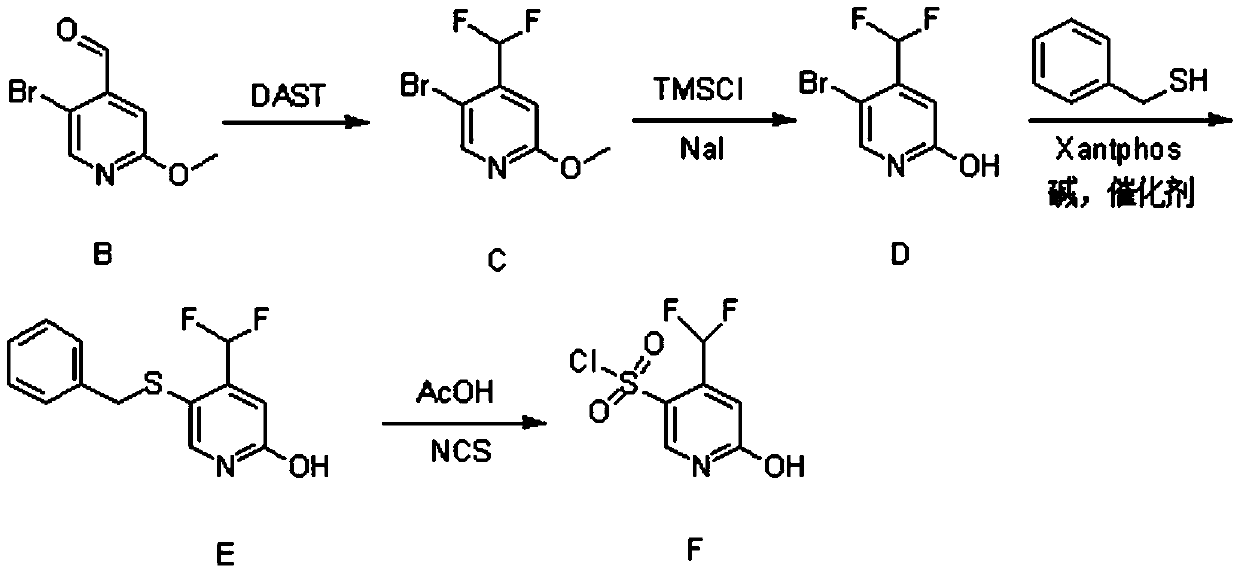
[0026] (1) Synthesis of Compound B
[0027] 5-Bromo-2-methoxypyridine (23g, 120mmol, 1eq.) was dissolved in 250ml of anhydrous tetrahydrofuran and set aside. Diisopropylamine (13.6g, 132mmol, 1.1eq.) was dissolved in 100ml of anhydrous tetrahydrofuran, cooled to -5°C, under nitrogen protection, n-butyl lithium (52.8ml, 132mmol, 1.1eq.) was added dropwise into the above solution. After completion of the dropwise addition, the mixture was stirred for 30 minutes.
[0028] The temperature of the above reaction system was lowered to -78°C, and a tetrahydrofuran solution of 5-bromo-2-methoxypyridine was added dropwise. After completion of the dropwise addition, the mixture was stirred for 1 hour. Then, N,N-dimethylformamide (13.4 g, 180 mmol, 1.5 eq.) was added dropwise to the above reaction system, and reacted for 2 hours.
[0029] After the reaction finished, slowly add 120ml of ammonium chloride solution in the reaction solution. It was extracted with n-hexane, and the organ...
Embodiment 2
[0044] (1) Synthesis of Compound B
[0045] 5-Bromo-2-methoxypyridine (28.8g, 150mmol, 1eq.) was dissolved in 300ml of anhydrous tetrahydrofuran and set aside. Diisopropylamine (17g, 165mmol, 1.1eq.) was dissolved in 150ml of anhydrous tetrahydrofuran, cooled to -5°C, under nitrogen protection, n-butyl lithium (66ml, 165mmol, 1.1eq.) was added dropwise to the above in solution. After completion of the dropwise addition, the mixture was stirred for 30 minutes.
[0046]The temperature of the above reaction system was lowered to -78°C, and a tetrahydrofuran solution of 5-bromo-2-methoxypyridine was added dropwise. After completion of the dropwise addition, the mixture was stirred for 1 hour. Then, N,N-dimethylformamide (16.8 g, 225 mmol, 1.5 eq.) was added dropwise to the above reaction system, and reacted for 2 hours.
[0047] After the reaction finished, slowly add 150ml ammonium chloride solution in the reaction solution. It was extracted with n-hexane, and the organic ph...
Embodiment 3
[0062] (1) Synthesis of Compound B
[0063] 5-Bromo-2-methoxypyridine (17.3g, 90mmol, 1eq.) was dissolved in 200ml of anhydrous tetrahydrofuran and set aside. Diisopropylamine (10.2g, 99mmol, 1.1eq.) was dissolved in 80ml of anhydrous tetrahydrofuran, cooled to -5°C, under nitrogen protection, n-butyl lithium (39.6ml, 99mmol, 1.1eq.) was added dropwise into the above solution. After completion of the dropwise addition, the mixture was stirred for 30 minutes.
[0064] The temperature of the above reaction system was lowered to -78°C, and a tetrahydrofuran solution of 5-bromo-2-methoxypyridine was added dropwise. After completion of the dropwise addition, the mixture was stirred for 1 hour. Then, N,N-dimethylformamide (10.1 g, 135 mmol, 1.5 eq.) was added dropwise to the above reaction system, and reacted for 2 hours.
[0065] After the reaction finished, slowly add 100ml of ammonium chloride solution in the reaction solution. It was extracted with n-hexane, and the organic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com