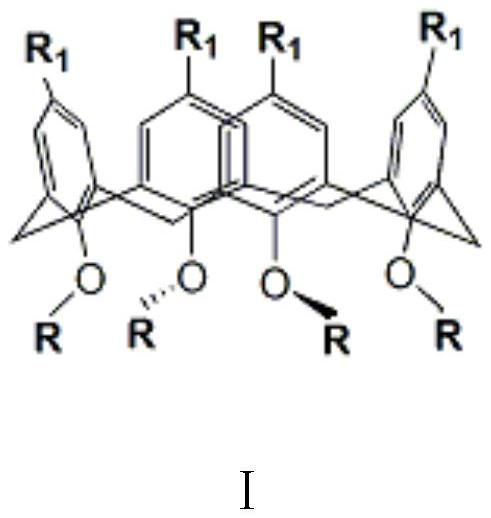
Calixarene Phase Transfer Catalyst and Its Application in Prothioconazole Intermediate Production
A technology of phase transfer catalyst and prothioconazole, which is applied in the direction of catalytic reaction, physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, etc., which can solve the problem of unsatisfactory materials, high cost and unfavorable industrialization Application and other issues, to achieve the effect of saving equipment investment, reducing decomposition, good molecular recognition and inclusion ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The structural formula of raw material 3,5-dichloro-2-pentanone is shown in IV:
[0045]
[0046] The structural formula of 1-acetyl-1-chlorocyclopropane is shown in V:
[0047]
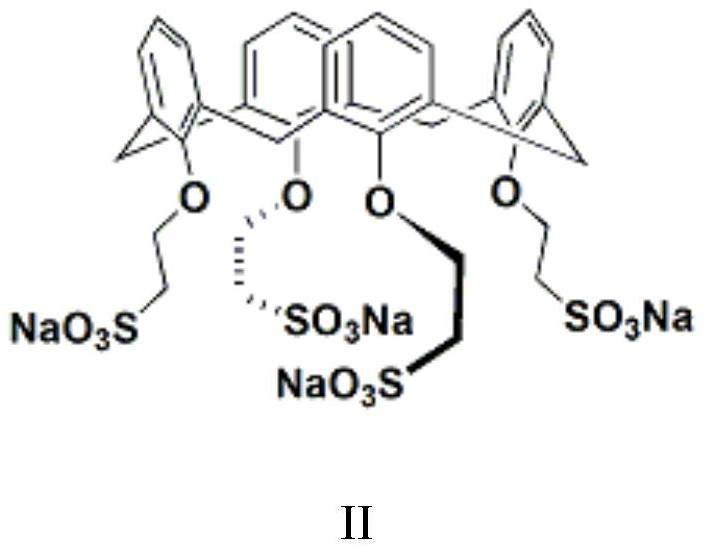
[0048] Set up mechanical stirring, reflux condenser and constant pressure dropping funnel above the 2L three-necked flask. At room temperature, add 500 mL of dichloromethane, 48 g (1.2 mol) of sodium hydroxide, 110 mL of water and 1.0 g of a calix[4]arene derivative phase transfer catalyst (structural formula shown in II). 155 g (1.0 mol) of 3,5-dichloro-2-pentanone was added within 45 min to the system at 40°C. Subsequently, at 40 °C. The reaction was continued for 1 hour, the system was lowered to room temperature, and the liquid was separated. After the organic phase was dried over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure, and the distillation was continued to obtain a light yellow oily liquid, which was the target product 1-acetyl-1-chlorocy...
Embodiment 2
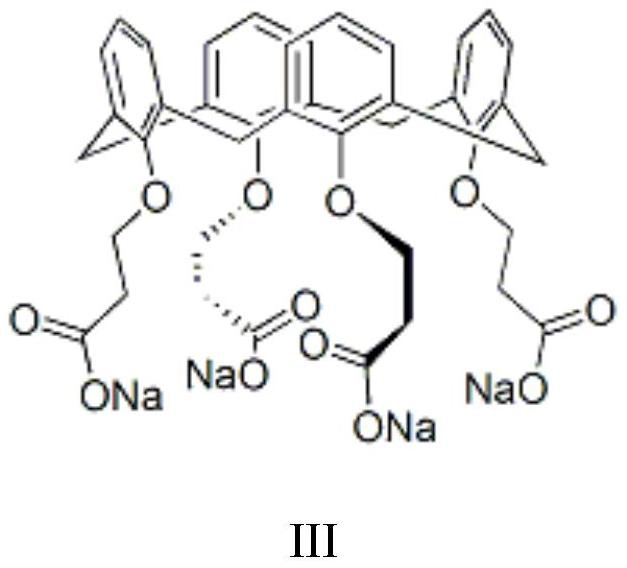
[0050] Set up mechanical stirring, reflux condenser and constant pressure dropping funnel above the 2L three-necked flask. At room temperature, 500 mL of chlorobenzene, 48 g (1.2 mol) of sodium hydroxide, 110 mL of water and 1.0 g of a calix[4]arene derivative phase transfer catalyst (structural formula as shown in III) were added. 155 g (1.0 mol) of 3,5-dichloro-2-pentanone was added within 45 min to the system at 40°C. Subsequently, at 40 °C. The reaction was continued for 1 hour, the system was lowered to room temperature, and the liquid was separated. After the organic phase was dried over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure, and the distillation was continued to obtain a light yellow oily liquid, which was the target product 1-acetyl-1-chlorocyclopropane , yield 96%, GC content > 98%.
[0051] The aqueous phase obtained by the reaction is acidified with hydrochloric acid until the pH is about 3.0, extracted with chlorobenzen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com