Method for synthesizing econazole nitrate by using one-pot method
A technology of econazole nitrate and imidazole, which is applied in the field of one-pot synthesis of econazole nitrate, which can solve the problems of easy harm to people and the environment, difficult storage of metal sodium, and cumbersome operation procedures, so as to reduce pollution and reduce solvent recovery The number of times, the effect of improving the reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
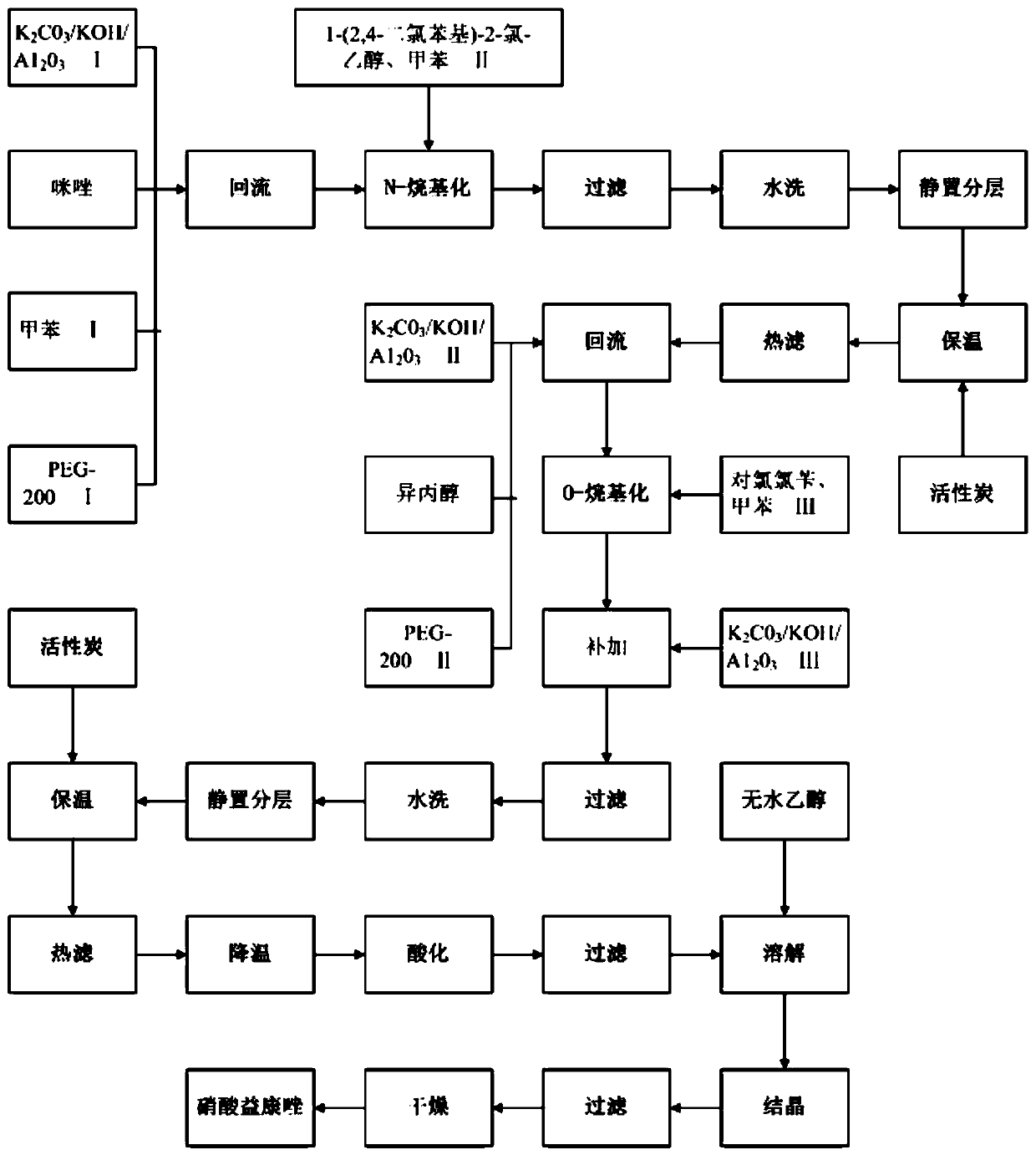
[0026] A method for the one-pot synthesis of econazole nitrate, such as figure 1 shown, including the following steps:
[0027] (1)K 2 CO 3 / KOH / A1 2 o 3 Preparation: 90g K 2 CO 3 , 35g KOH dissolved in 110g water, add 145gA1 2 o 3 , with stirring at 60°C, heat preservation for 1h, rotary evaporation, and drying at 120°C for 4h to obtain 258.7g K 2 CO 3 / KOH / A1 2 o 3 ;
[0028] (2) preparation of econazole nitrate: 80gK 2 CO 3 / KOH / A1 2 o 3 Ⅰ, 30g imidazole, 190g toluene Ⅰ, 2.5g PEG-200Ⅰ were added to a 500mL reaction bottle, the temperature was raised to reflux and the water was separated for 30 minutes, the temperature was lowered to 60°C, and 90g 1-(2,4-dichlorophenyl)-2-chloro - Mixed solution of ethanol and 120g toluene II, heated to 72°C, reacted for 3.5h; filtered, washed with water, combined the filtrate and washing liquid, left to separate the organic layer, washed the organic layer with water once, added 0.5g activated carbon I, Insulated for 10 minut...
Embodiment 2
[0031] A method for synthesizing econazole nitrate in one pot, comprising the following steps:
[0032] (1)K 2 CO 3 / KOH / A1 2 o 3 Preparation: 90g K 2 CO 3 , 35g KOH dissolved in 110g water, add 145gA1 2 o 3 , with stirring at 65°C, heat preservation for 1h, rotary evaporation, and drying at 120°C for 4h to obtain 257.6g K 2 CO 3 / KOH / A1 2 o 3 ;
[0033] (2) preparation of econazole nitrate: 90gK 2 CO 3 / KOH / A1 2 o 3 Ⅰ, 29.5g imidazole, 180g toluene Ⅰ, 1.6g PEG-200Ⅰ were added to a 500mL reaction bottle, the temperature was raised to reflux and water was separated for 25 minutes, the temperature was lowered to 60°C, and 90g 1-(2,4-dichlorophenyl)-2-chloro -The mixture of ethanol and 130g of toluene II was heated up to 71°C and reacted for 3.0h. Filtrate, wash with water, combine the filtrate and washing liquid, let stand to separate the organic layer, wash the organic layer once with water, add 0.7g of activated carbon I, keep warm for 10 minutes, heat filter, ...
Embodiment 3
[0036] A method for synthesizing econazole nitrate in one pot, comprising the following steps:
[0037] (1)K 2 CO 3 / KOH / A1 2 o 3 Preparation: 90g K 2 CO 3 , 35g KOH dissolved in 110g water, add 145gA1 2 o 3 , with stirring at 62°C, heat preservation for 1h, rotary evaporation, and drying at 120°C for 4h to obtain 256.5g K 2 CO 3 / KOH / A1 2 o 3 ;
[0038] (2) preparation of econazole nitrate: 95gK 2 CO 3 / KOH / A1 2 o 3 Ⅰ, 28.6g imidazole, 170g toluene Ⅰ, 3.2g PEG-200Ⅰ were added to a 500mL reaction bottle, the temperature was raised to reflux for 35 minutes, the temperature was lowered to 60°C, and 90g 1-(2,4-dichlorophenyl)-2-chloro- The mixture of ethanol and 140g of toluene II was heated to 73°C and reacted for 3.5 hours; filtered, washed with water, combined with the filtrate and washing liquid, left to separate the organic layer, washed the organic layer with water once, added 0.9g of activated carbon Ⅰ, and kept warm 10min, heat filtration, obtain the imida...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com