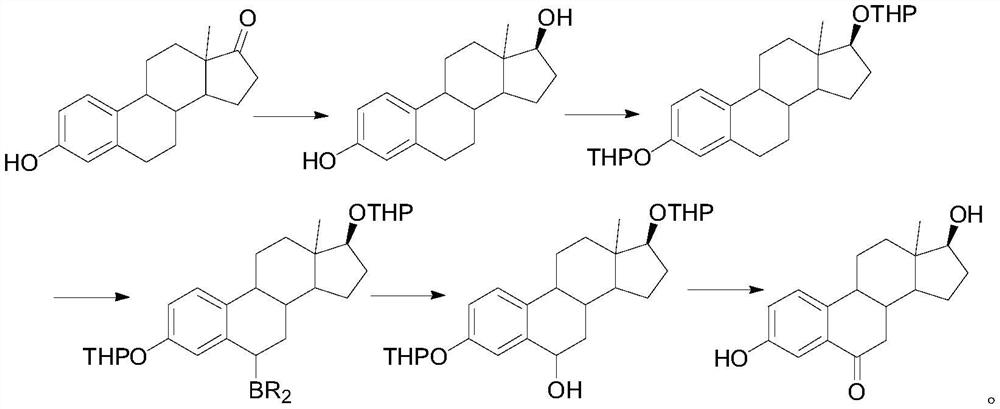
A kind of synthetic method of 6-ketoestradiol
A technology for the synthesis of ketone estradiol, which is applied in the direction of chemical instruments and methods, steroids, estrone derivatives, etc., can solve the problems of increased production difficulty, high production risk, long steps, etc., and achieve cost Low cost, green reaction conditions and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Reduction reaction
[0023] In the reaction flask, add 50 g of compound 1, add 100 mL of THF and 100 mL of methanol to dissolve, cool down to 0-10 °C, add 5 g of sodium borohydride, keep stirring for 2 h, TLC shows that the reaction is complete, add 10 mL of glacial acetic acid dropwise to quench the reaction, add 100 mL of water, the system was concentrated under reduced pressure at 50 °C until solvent-free, the solid was precipitated, cooled to room temperature, stirred for 1 h, filtered, and the filter cake was washed with water and dried to obtain compound 2 with a yield of 96% and a purity of 98%.
[0024] 2. Oxidation reaction
[0025] In the reaction flask, add 30 g of compound 2, 100 mL of propylene acetate, and 3 g of p-toluenesulfonyl chloride p-TS. Under nitrogen protection, heat to 60 °C for 6 h. After the reaction is completed, the propylene acetate is concentrated under reduced pressure, and 200 mL of acetic acid is added. Ethyl ester, lowered and warm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com