Bromfenac sodium eye drops and preparation process thereof
The technology of bromfenac sodium and preparation process is applied in the field of bromfenac sodium eye drops and its preparation technology, which can solve the problems of poor sealing performance of a stirring device, reduced drug efficacy, and inability to perform heating, and achieves good physical and mechanical properties. Stability, good biocompatibility, the effect of increasing the speed of stirring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
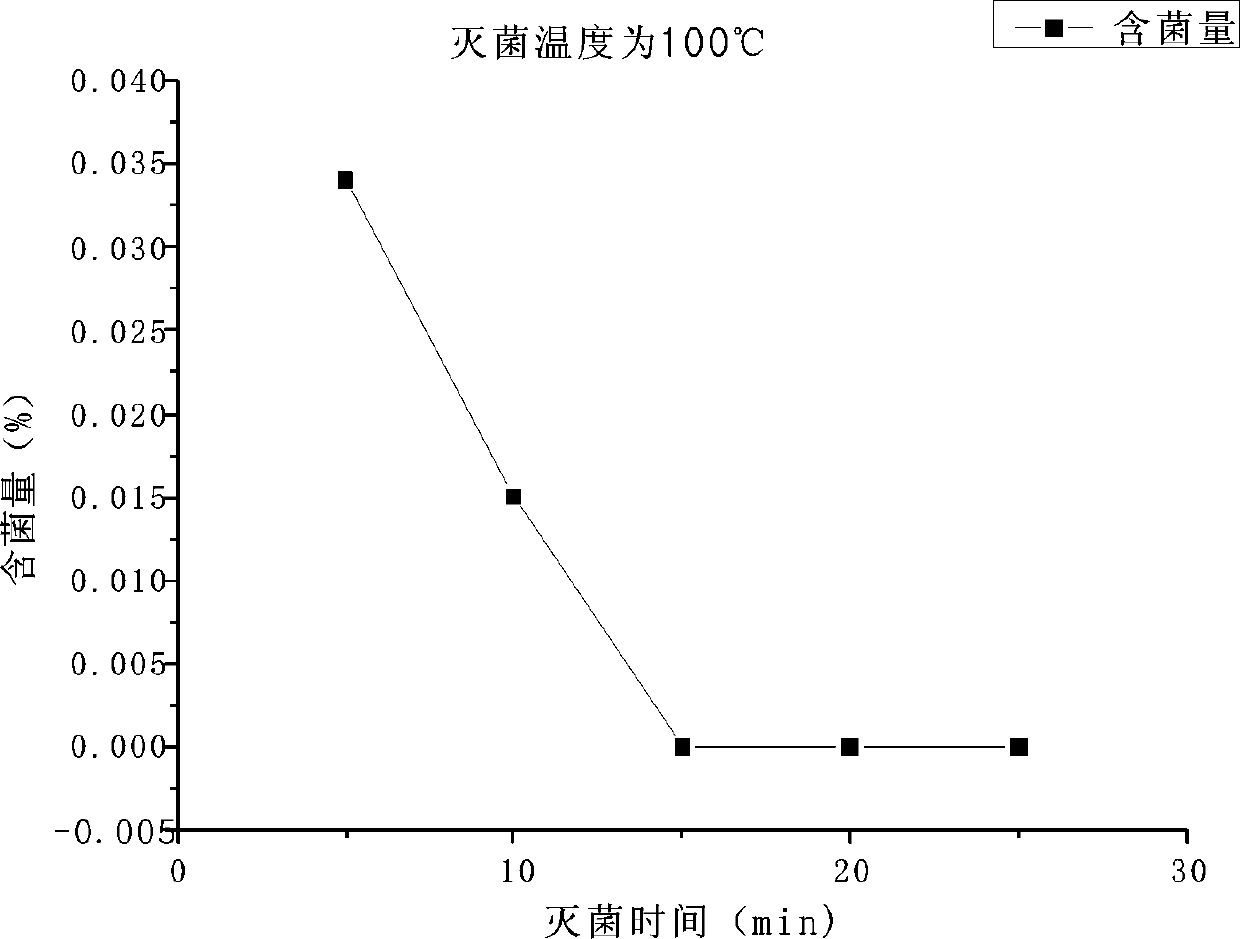
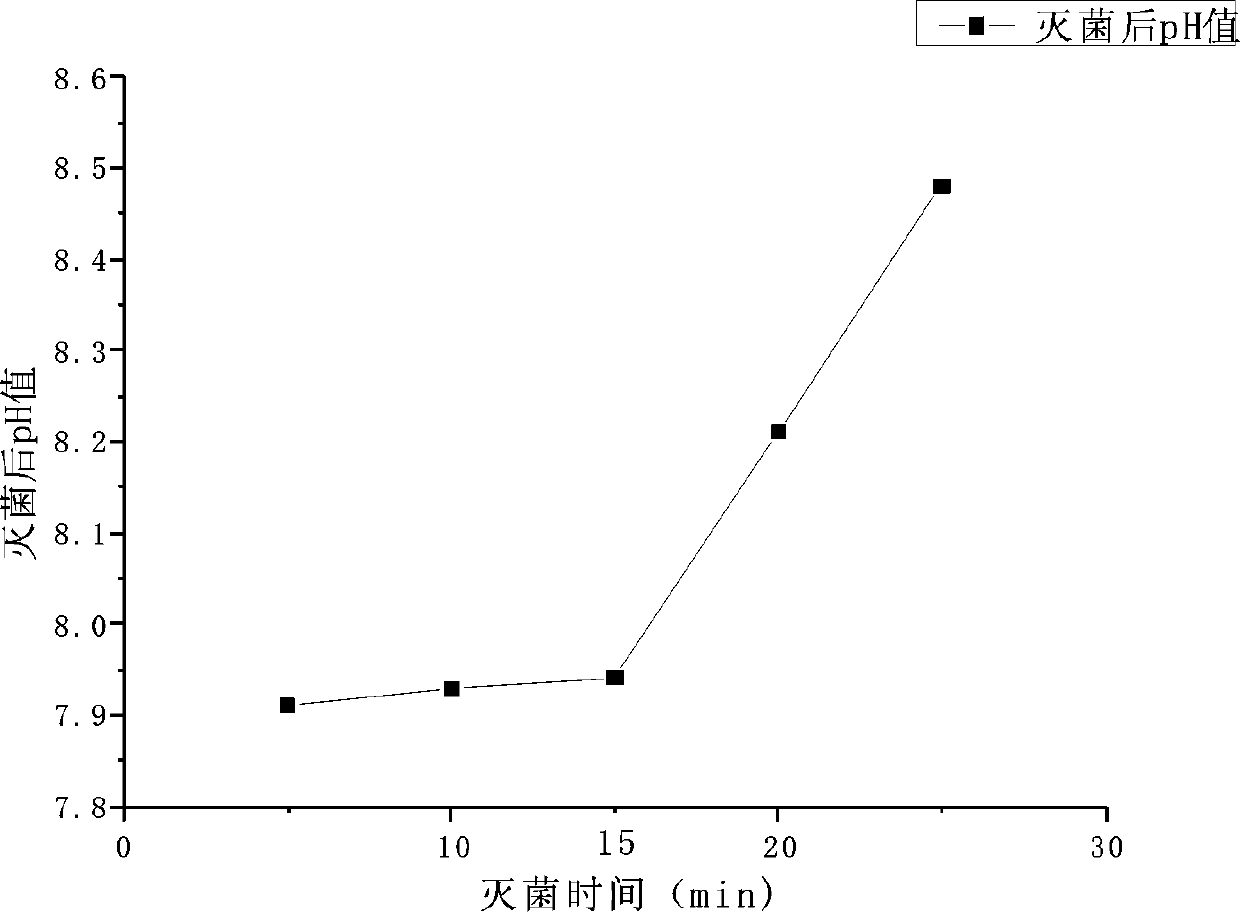
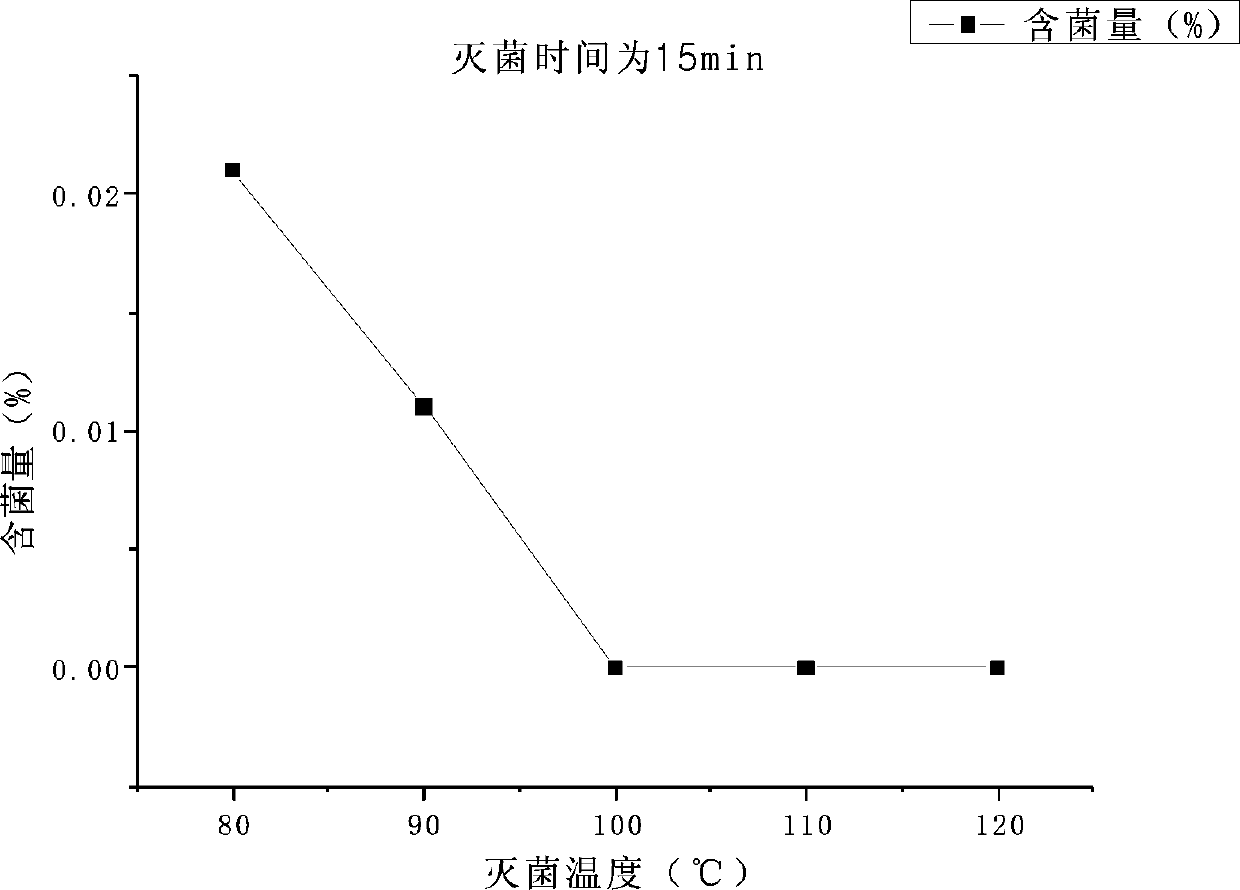
[0077] The present invention is described in further detail now in conjunction with accompanying drawing. These drawings are all simplified schematic diagrams, which only illustrate the basic structure of the present invention in a schematic manner, so they only show the configurations related to the present invention.
[0078] A kind of bromfenac sodium eye drops, each percentage by weight proportioning composition is shown in Table 1:
[0079] Table 1 bromfenac sodium eye drops embodiment 1-4
[0080]
[0081] The preparation technology of described bromfenac sodium eye drops, comprises the steps:
[0082] Step 1: Add 0.06% by weight thickener povidone into the stirring device, add an appropriate amount of water for injection, start the stirring device and heat until the water temperature reaches 40°C-50°C, and stir for 2-3 minutes;
[0083] Step 2: Add 0.06% edetate disodium, 0.2% antioxidant: anhydrous sodium sulfite, 0.1% bacteriostatic agent: methylparaben, 0.2% bro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com