Compound, organic light-emitting device and display device
A compound and connection position technology, applied in the field of organic electroluminescence, can solve the problems of device electron and hole mobility imbalance, device efficiency and lifetime reduction, electron transport performance reduction, etc., to avoid degradation or attenuation, low driving voltage , excellent film stability and uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
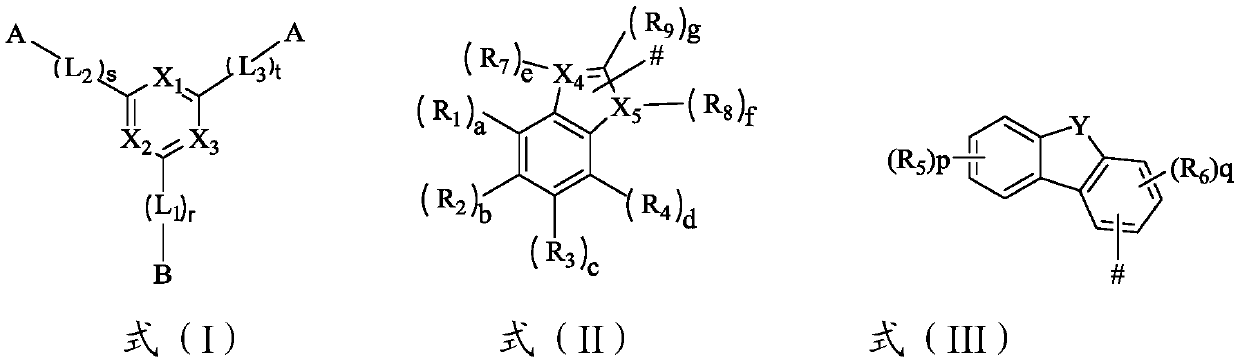
[0084] According to one embodiment of the present invention, the compound has the structure shown in formula (I-1),
[0085]
[0086] Among them, X 1 -X 3 Each independently selected from N atom or C atom; L 1 -L 3 each independently selected from substituted or unsubstituted C5-C40 aryl, substituted or unsubstituted C3-C40 heteroaryl; r, s, t each independently selected from 0 or 1; Y selected from N atom, O atom or S atom.
[0087] According to an embodiment of the present invention, r, s, and t are all 0.
[0088] According to one embodiment of the present invention, R 1 -R 9 Each independently selected from substituted or unsubstituted C5-C40 aryl, substituted or unsubstituted C3-C40 heteroaryl, substituents selected from C1-C10 alkyl, C3-C10 cycloalkyl, C2-C10 Any one of alkenyl, C1-C6 alkoxy, halogen, cyano, C6-C30 single-ring aromatic hydrocarbon or condensed-ring aromatic hydrocarbon group, C3-C30 single-ring heteroaromatic hydrocarbon or condensed-ring heter...
preparation example 1
[0104] Preparation of Preparation Example 1 Compound HB01
[0105]
[0106] The preparation method is as follows:
[0107] (1) Preparation of compound HB01-1:
[0108] In a 250 mL round bottom flask, 1,3-dibromo-5-iodobenzene (10 mmol), copper iodide (15 mmol), potassium tert-butoxide (65 mmol), 1,2-diaminocyclohexane (12 mmol) and 2-phenyl-1H-benzimidazole (25mmol) were added to dry 1,4-dioxane (150mL), refluxed under nitrogen atmosphere for 48 hours, the obtained intermediate was cooled to room temperature, added to water, It was then filtered through a pad of celite, and the filtrate was extracted with dichloromethane, then washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude product was purified by silica gel column chromatography to obtain intermediate product HB01-1.
[0109] (2) Preparation of compound HB01:
[0110] In a 250 mL round bottom flask, the intermediate product HB03-1, copper iodide (15 mmol), potas...
preparation example 2
[0112] Preparation of Preparation Example 2 Compound HB18
[0113]
[0114] The preparation method is as follows:
[0115] (1) Preparation of compound HB18-1:
[0116] In a 250 mL round bottom flask, 1,3-dibromo-5-iodobenzene (10 mmol), 1-benzonitrile-2-boronate-benzimidazole (22 mmol) and Pd(PPh 3 )4 (0.3mmol) was added to a mixture of toluene (30mL) / ethanol (20mL) and potassium carbonate (12mmol) aqueous solution (10mL), and refluxed under nitrogen atmosphere for 12h. The resulting mixture was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude product was purified by silica gel column chromatography The final product HB18-1 was obtained.
[0117] (2) Preparation of compound HB18:
[0118] In a 250 mL round bottom flask, the intermediate product HB18-1, copper iodide (15 mmol), potassi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com