Method for eliminating HRP (Horseradish Peroxidase) antibody interference
A technology of capture method and enzyme-linked immunoassay, which is applied in the field of in vitro diagnosis and can solve problems such as interference with test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1. Effect of adding apo-HRP on TORCH-IgM detection results
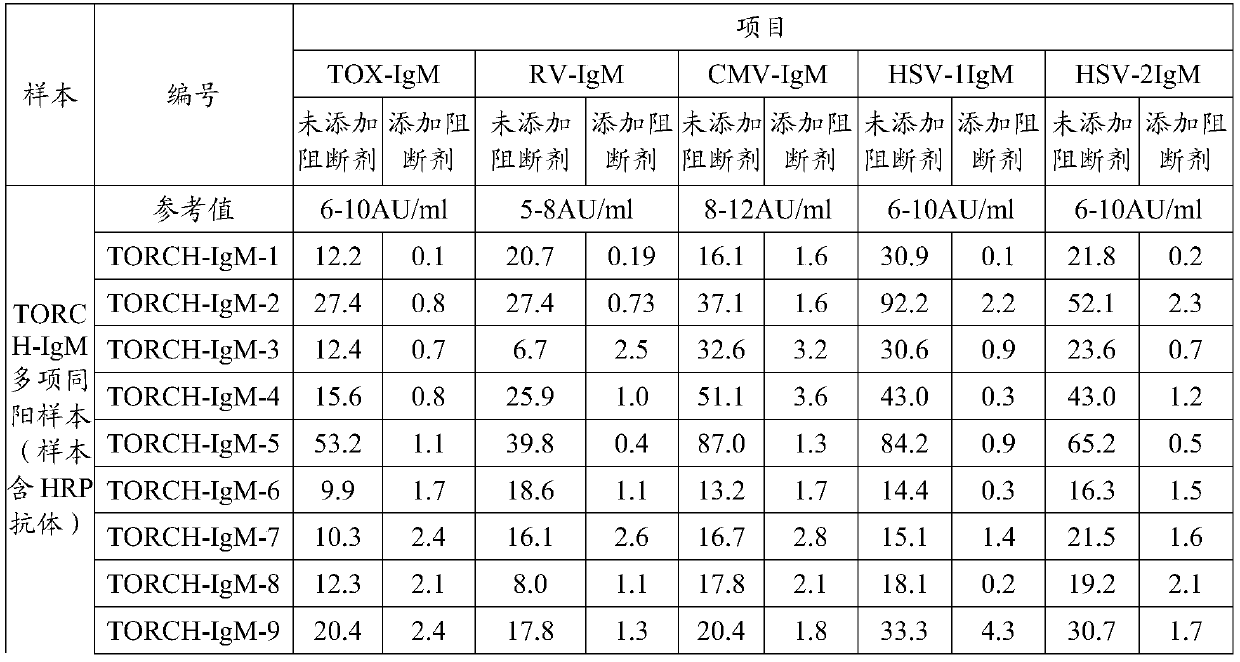
[0024] 25 samples to be tested were detected by magnetic particle TORCH-IgM (TOX / RV / CMV / HSV-1 / HSV-2 IgM) 5-item detection kit (Antu Bio). In addition, use the magnetic particle TORCH-IgM kit (Antu Biology) with a blocking agent (0.5 mg / ml apo-inactivated HRP) added to the enzyme conjugate or sample diluent to simultaneously detect the above 25 samples to be tested. The results are shown in Table 1.
[0025] Table 1 compares the effect of adding apo-HRP on the detection results of TORCH-IgM
[0026]
[0027]
[0028] The results showed that after adding 0.5mg / ml apo-inactivated HRP, the false positives of TORCH-IgM multi-positive samples (containing HRP antibody in the samples) were eliminated, while the detection results of normal samples were not affected.
Embodiment 2
[0029] Example 2. Adding apo-HRP eliminates the interference of HRP antibody to the competition method
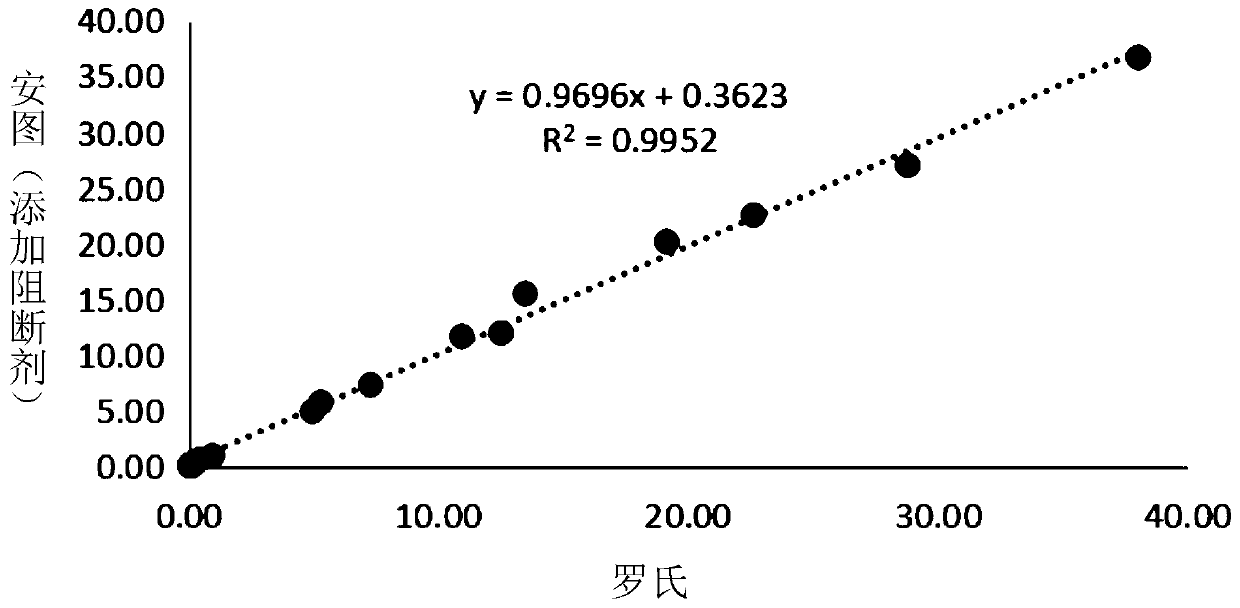
[0030] The progesterone quantitative detection kit (magnetic particle chemiluminescence method) of Antu Bio and the progesterone detection kit (electrochemiluminescence method) of Roche were used to detect 16 samples to be tested. In addition, the magnetic particle progesterone quantitative detection kit (Antu Biology) added with a blocking agent (0.01mg / ml apo-HRP) to the enzyme conjugate was used to simultaneously detect the above 16 samples to be tested. The test results are shown in Table 2 and Figure 1-2 .
[0031] Table 2 compares the effect of adding apo-HRP on the detection results of the competition method
[0032]
[0033]
[0034] The results showed that compared with the results detected by the progesterone quantitative detection kit (magnetic particle chemiluminescence method) without adding blocking agent, the amount detected by the progesterone quanti...
Embodiment 3
[0036] 1. Verification of the HRP antibody contained in the sample 1
[0037] The aprosthetic group-inactivated HRP was coated on the microtiter plate, after washing, 25 samples to be tested were added, after incubation and washing, enzyme-labeled anti-antibodies (anti-human IgG antibody, anti-human IgM antibody) were added for detection by indirect method IgG and IgM antibodies in the sample (IgM antibody detection: the sample is pre-adsorbed with goat anti-human IgG), the experimental data is as follows:
[0038] Table 3: Sample containing HRP antibody validation experiment 1
[0039]
[0040]
[0041]The results showed that there was no antibody against inactivated HRP in normal samples, but antibodies against inactivated HRP were contained in TORCH-IgM positive samples.
[0042] 2. Verification of the HRP antibody contained in the sample 2
[0043] Use the magnetic particle CMV-IgM antibody detection kit to detect 20 samples to be tested, add 0.01mg / ml HRP (with bi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com