Preparation of novel viologen electrochromic material and application thereof
An electrochromic material and electrochromic technology, applied in the direction of color-changing fluorescent materials, instruments, organic chemistry, etc., can solve the problems of reducing electrochromic voltage, complex synthesis process, single color type, etc., and achieve low color-changing voltage. Good stability and wide range of discoloration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
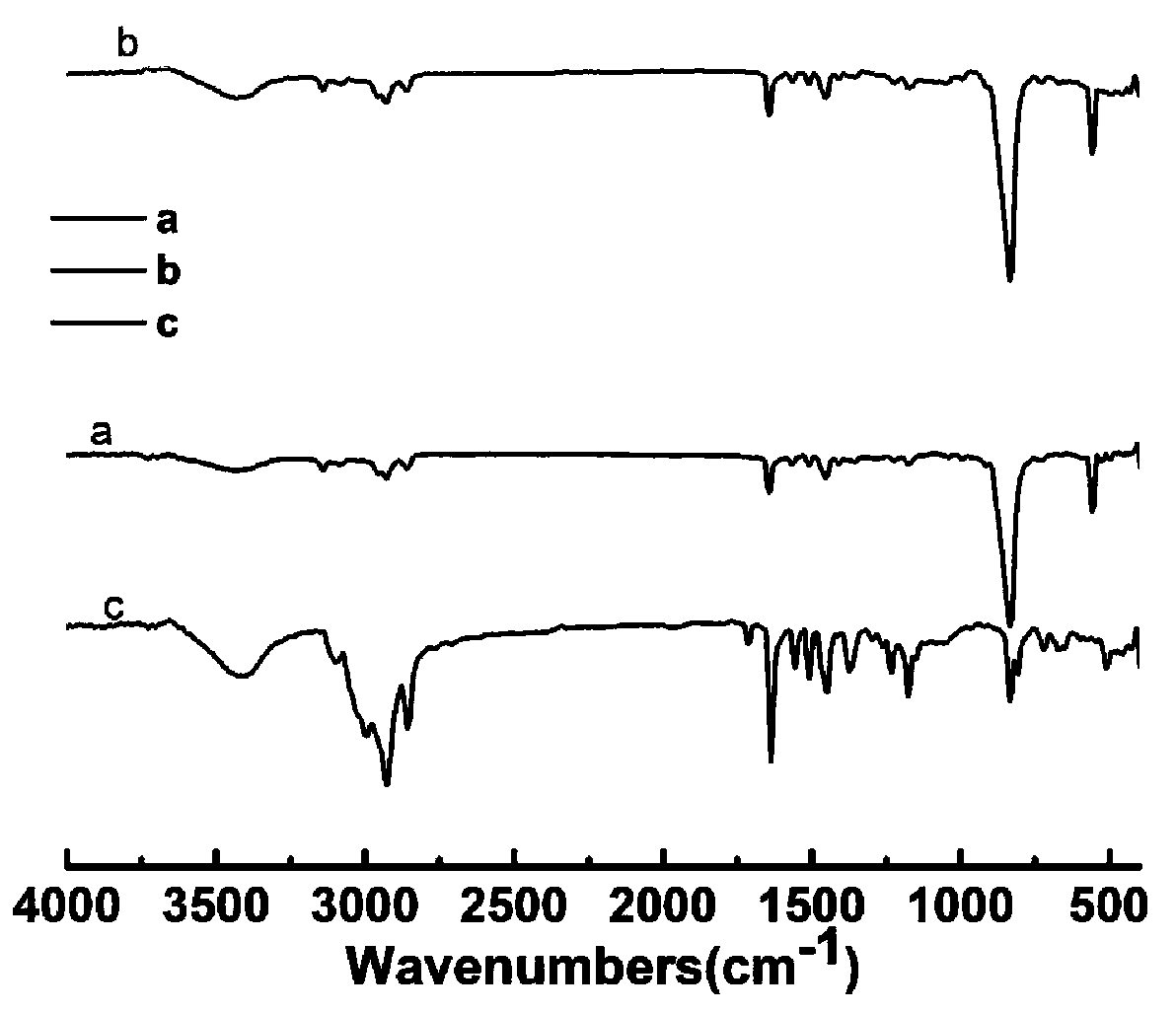
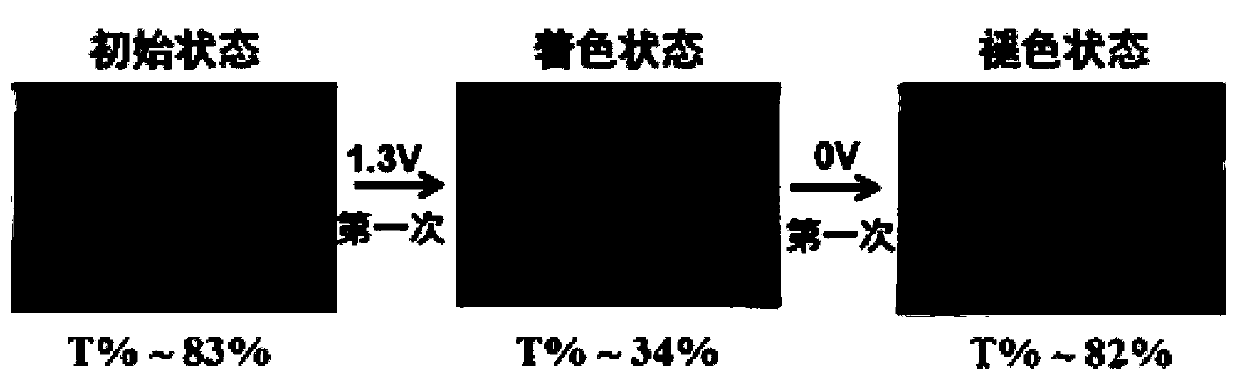
Image
Examples
Embodiment 1
[0035] The present embodiment provides a kind of viologen electrochromic material formula (1), and its preparation method comprises the following steps:
[0036] (1) Dissolve 1.825g of 4,4-bipyridine in 10ml of ACN, slowly drop into it 2g of 1-bromoheptane solution diluted with 10ml of ACN, stir and react at 50°C for 72h, filter and wash with anhydrous ether, After vacuum drying at 50°C for 24 hours, 1-heptyl-4,4'-bipyridyl bromide (MHVBr) was obtained with a yield of 47%.
[0037] (2) 1.675 g of the solid product obtained in step (1) was dissolved in 10 ml of DMF, and 1.53 g of p-chloromethylstyrene solution diluted with 5 ml of DMF was slowly added dropwise thereto, and the reaction was stirred at 60° C. for 72 h, Filter and wash with anhydrous ether, and dry in vacuum at 50°C for 24 hours to obtain 1-heptyl-1-[4'-(vinyl)benzyl]-4,4'-bipyridyl chloride bromide (HBVBrCl), product The rate is 60%.
[0038] (3) Dissolve 0.5 g of 1-heptyl-1-[4-(vinyl)benzyl]-4,4'-bipyridyl chl...
Embodiment 2
[0043] The present embodiment provides a kind of viologen electrochromic material formula (2), and its preparation method comprises the following steps:
[0044] (1) Dissolve 1.825g of 4,4-bipyridine in 10ml of ACN, slowly drop into it 2g of 1-bromoheptane solution diluted with 10ml of ACN, stir and react at 50°C for 72h, filter and wash with anhydrous ether , dried under vacuum at 50°C for 24 hours to obtain 1-heptyl-4,4'-bipyridyl bromide with a yield of 47%.
[0045] (2) Dissolve 0.9 g of the solid product obtained in step (1) in 10 ml of DMF, slowly drop into it 0.33 g of 1,6-dibromohexane solution diluted with 5 ml of DMF, and stir the reaction at 60° C. for 72 h, Filter and wash with anhydrous ether, and dry at 50°C for 24 hours to obtain 1-heptyl-1-[1-hexyl-1-heptyl-4,4'-bipyridyl]-4,4'-bipyridyl tetrabromide , the yield was 36%.
[0046] (3) 0.3g of 1-heptyl-1-[1-hexyl-1-heptyl-4,4'-bipyridyl]-4,4'-bipyridyl tetrabromide prepared in step (2) Dissolve in 5ml deionize...
Embodiment 3
[0051] The present embodiment provides a kind of viologen electrochromic material formula (3), and its preparation method comprises the following steps:
[0052] (1) Dissolve 1.825g of 4,4-bipyridine in 10ml of ACN, slowly drop into it 2g of 1-bromoheptane solution diluted with 10ml of ACN, stir and react at 50°C for 72h, filter and wash with anhydrous ether, After vacuum drying at 50°C for 24 hours, 1-heptyl-4,4'-bipyridyl bromide (MHVBr) was obtained with a yield of 47%.
[0053] (2) 1.675 g of the solid product obtained in step (1) was dissolved in 10 ml of DMF, and 1.53 g of p-chloromethylstyrene solution diluted with 5 ml of DMF was slowly added dropwise thereto, and the reaction was stirred at 60° C. for 72 h, Filter and wash with anhydrous ether, and dry in vacuum at 50°C for 24 hours to obtain 1-heptyl-1-[4'-(vinyl)benzyl]-4,4'-bipyridyl chloride bromide (HBVBrCl), product The rate is 60%.
[0054] (3) Dissolve 1 g of 1-heptyl-1-[4-(vinyl)benzyl]-4,4'-bipyridyl chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com