Glipizide tablets and application thereof
A technology of glipizide tablets and glipizide, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pill delivery, etc., and can solve the problem of lowering blood sugar by glipizide and arctiin There are no other problems, and the product has less impurities, excellent effect and good uniformity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
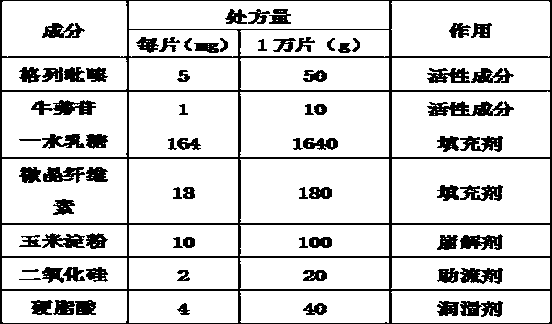
[0023] A glipizide tablet, its prescription is shown in Table 1.
[0024] Table 1
[0025]
[0026] Tablet preparation process:
[0027] ①Pretreatment of raw and auxiliary materials:
[0028] Receive qualified raw materials and auxiliary materials, pass stearic acid through an 80-mesh sieve, and set aside.
[0029] ②Weighing:
[0030] Weigh glipizide, arctiside, lactose monohydrate, microcrystalline cellulose, cornstarch, stearic acid according to the recipe quantity, for subsequent use;
[0031] ③Mix:
[0032] Mix 50g of glipizide and lactose monohydrate in equal amounts (about 3 times) (100g+200g+400g), each time for 5 minutes, and place the remaining amount of lactose monohydrate together in a three-dimensional motion Mixing was carried out in a mixer, rotating speed: 12 rpm, mixing time: 1 h.
[0033] Take it out, pass through a 30-mesh sieve to remove agglomerates, and then place it in a three-dimensional motion mixer to mix for 3 hours. Add 10 g of arctiside, 1...
Embodiment 2
[0039] Sample quality and dissolution testing.
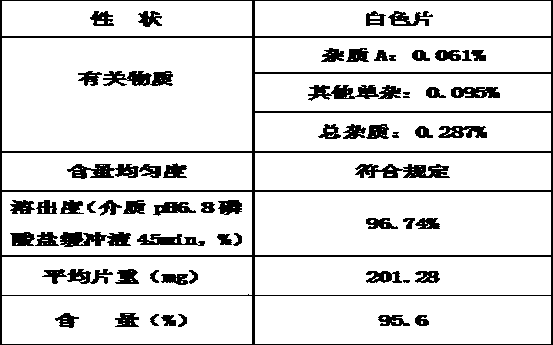
[0040] The product of the present invention is tested for correlation, content and dissolution. The specific data are as follows 2:
[0041] Table 2 Test results of finished products
[0042]
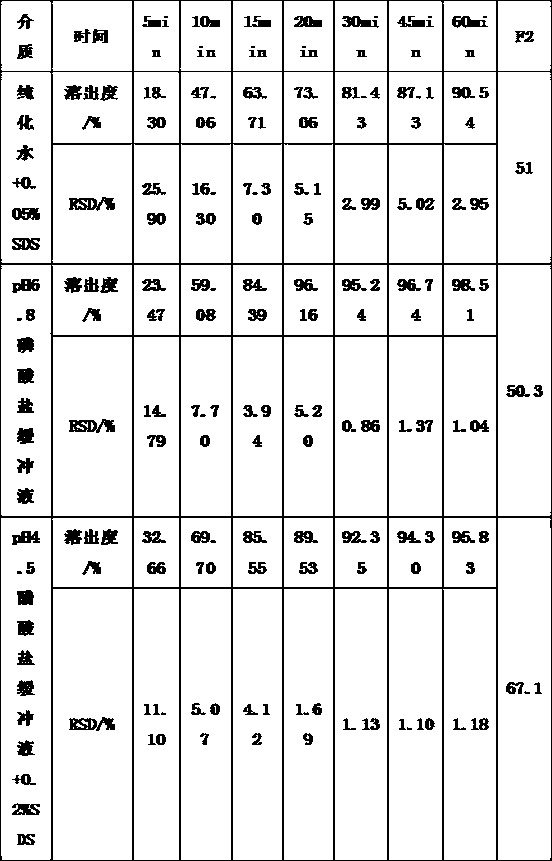
[0043] Table 3 Dissolution curve results of finished products in four media
[0044]
Embodiment 3
[0050] Animal experiment.
[0051] Animal grouping: 40 Kunming strain mice were selected, half male and half female, weighing 19.4±2.5g.
[0052] The blank control group, 5 males and 5 males, was given the same amount of normal saline without modeling;
[0053] Model control group: 5 males and 5 males, no drug for modeling, but the same amount of normal saline;
[0054] Comparative example group: 5 males and 5 males, modeled and given the drug of Comparative Example 1;
[0055] Experimental group: 5 males and 5 males, modeled and given the medicine of Example 1.
[0056] Modeling of diabetic mice: The mice were fasted for 12 hours and injected intraperitoneally with 100 mg / kg of alloxan.
[0057] Administration: After 2 days of modeling, the rats were given continuous intragastric administration for 8 days, and the dosage was 3 mg / kg.
[0058] Blood glucose determination: After 1 h of administration on the 8th day, 1 mL of blood was collected from the inner canthus of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com