Method for detecting content of threo beta-hydroxy-alpha-amino acid
An amino acid and hydroxyl technology, applied in the field of analysis and detection research, achieves the effect of simple and convenient operation and mild detection conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Dehydratase gene obtained
[0014] 1.1 Synthesis and acquisition of dehydratase gene
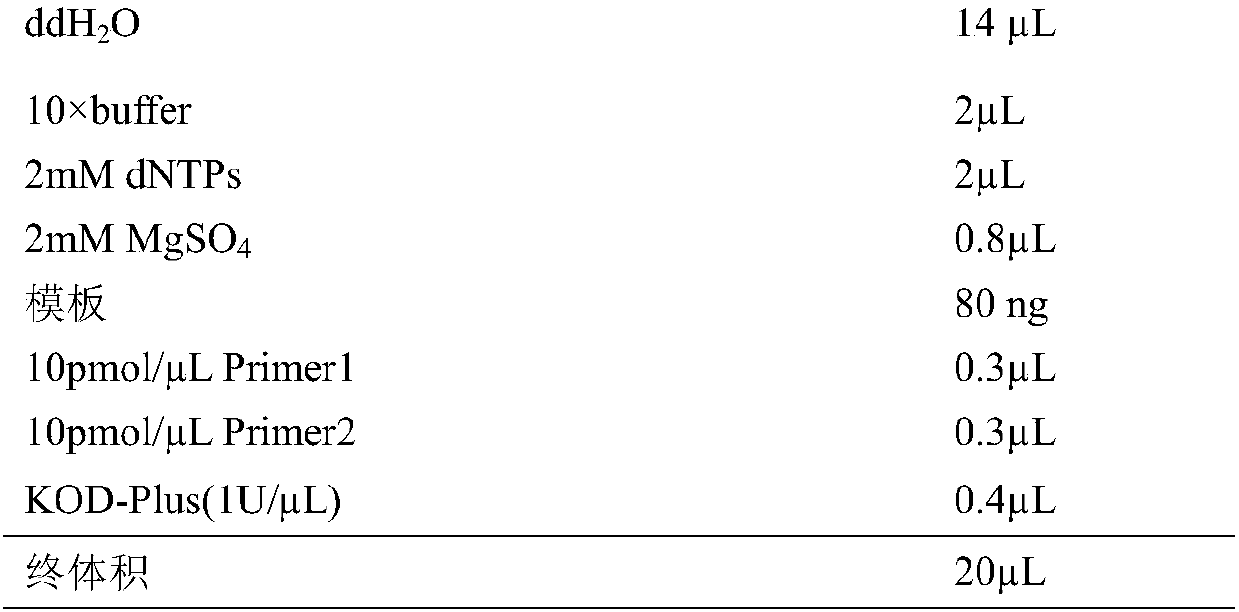
[0015] The dehydratase gene is derived from Paraburkholderia xenovorans LB400 (GenBank: AIP32383). According to the codon preference of Escherichia coli, the gene is optimized and sent to the company for synthesis. The codon-optimized genes are respectively added with NdeI (CATATG) enzyme cutting site at the 5' end point, add XhoI (CTCGAG) restriction site at the 3' end, and clone into PET21a vector. Using the PET21a plasmid with the RasADH gene as a template and designing primers according to the RasADH gene sequence for rolling circle amplification, the PCR amplification reaction system is:
[0016]
[0017] The PCR amplification conditions are:
[0018]
[0019] The PCR amplification products were purified and recovered using a DNA purification kit. The PCR product purified by the DNA purification kit was digested with Dpn I restriction endonuclease, and digeste...
Embodiment 2
[0028] Example 2: Construction and induced expression of dehydratase expression bacteria
[0029] Transfer the digested and purified PCR product into Escherichia coli BL21(DE3) competent cells by chemical transformation, pick a single clone into 4 mL LB medium containing ampicillin (1 mg / mL), and take fresh bacterial liquid Sent to the sequencing company for sequencing, and the sequencing results showed the correct expression bacteria. Prepare 50 mL of seed liquid, and the medium is LB liquid medium (peptone 10g / L, yeast powder 5g / L, NaCl 10g / L), pick a single colony of genetically engineered bacteria with an inoculation loop and insert it into the medium, 37°C, 200rpm Incubate overnight. The overnight cultured seed solution was transferred to the fermentation medium at an inoculum size of 1%, and cultured at 25° C. and 200 rpm for 20 h. Take 5 mL of the fermented liquid to concentrate and ultrasonically disrupt the bacteria, and detect the dehydratase activity.
Embodiment 3
[0030] Example 3: Dehydratase Purification
[0031] After the induced expression, the fermentation broth was centrifuged at 6500rpm for ten minutes to collect the bacterial cells, and the collected bacterial cells were resuspended and washed once with buffer (0.1mol / L pH 7.0 sodium phosphate buffer and 0.5M NaCl), and centrifuged in the same way. The suspension was crushed with a high-pressure homogenizer, and the crushed liquid was centrifuged at 6000 rpm at 4°C for 20 minutes to obtain a crude enzyme liquid. The obtained crude enzyme liquid was separated and purified using a His-Trap TM / FF affinity chromatography column, The eluent (0.1M sodium phosphate, 0.5M NaCl, 0.25M imidazole, pH 7.0) was used for gradient elution, the active fraction was collected, and the purity of the enzyme protein was detected by SDS-PAGE.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com