Pantoprazole sodium enteric mini-tablet capsule and preparation method thereof
A pantoprazole sodium and enteric-coated technology, which is applied in the field of pantoprazole sodium enteric-coated micro-tablet capsules and its preparation, can solve the problems of low bioavailability, complex preparation process, poor long-term storage stability, etc., and achieve simple process , stable quality, blood concentration consistent with the original research effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A preparation method for pantoprazole sodium enteric-coated microtablet capsules, comprising the following steps:
[0037] S1, the preparation method of described pantoprazole sodium tablet core comprises: first take by weighing pantoprazole sodium, Pearlitol 200SD, anhydrous sodium carbonate, PVPP, PVP.K30 and magnesium stearate cross 80-100 mesh sieve standby ; Then mix Pearlitol 200SD, anhydrous sodium carbonate, PVPP, PVP.K30 and magnesium stearate evenly for later use; then mix pantoprazole sodium with the above mixed powder in equal proportions; finally choose 3mm or 5mm, 7mm Punch, the hardness of the tablet is about 40N-60N, and the pantoprazole sodium tablet is prepared by powder direct compression or wet granulation process. In tablet compression, the surface of the tablet is smooth, no sticking, no cracks, and good fluidity.
[0038] S2, spray the prepared barrier layer on the pantoprazole sodium tablet core again, the preparation method of the barrier layer...
Embodiment 1
[0042] The invention provides a method, and each capsule is embedded with four pantoprazole sodium enteric-coated microtablets, calculated in 1000 tablets.
[0043] Pantoprazole sodium tablet core contains the following components:
[0044]
[0045] The isolation layer includes the following components:
[0046]
[0047]
[0048] Add water and be mixed with 5% solid content solution, and isolation layer accounts for 5% of the mass of pantoprazole sodium enteric-coated microtablet;
[0049] The enteric layer includes the following components:
[0050]
[0051] Water is added to prepare a solution with a solid content of 10%, and the enteric layer accounts for 10% of the mass of the pantoprazole sodium enteric-coated microtablets.
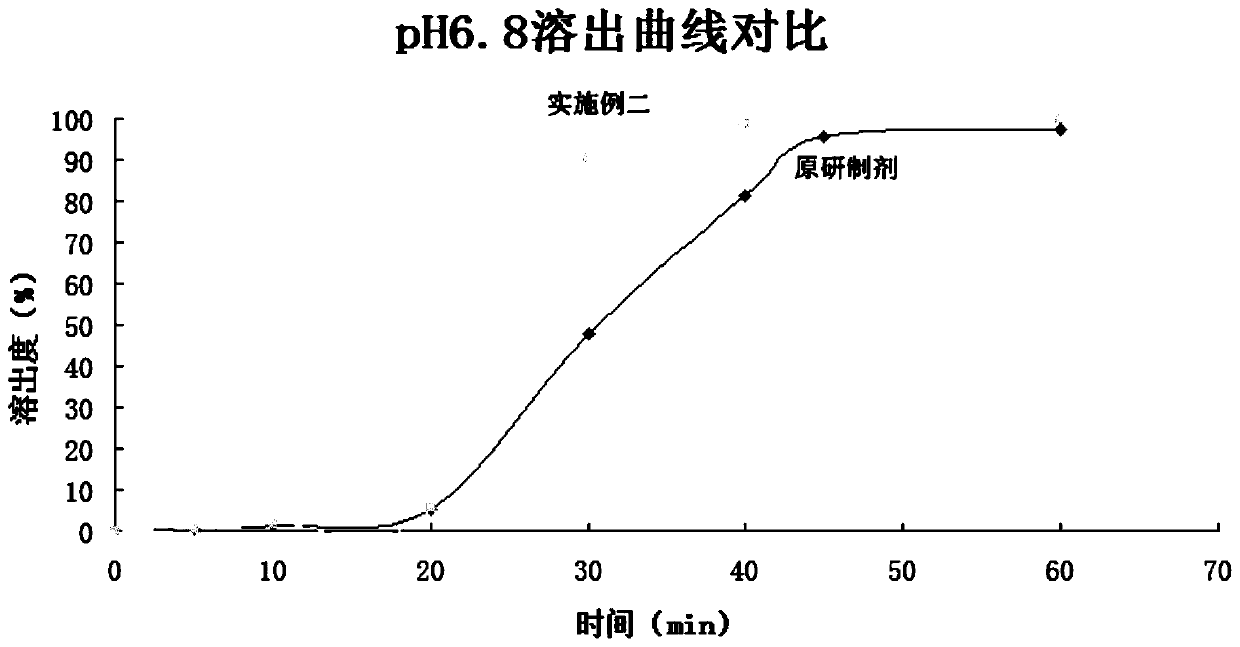
Embodiment 2
[0053] The invention provides a method, and each capsule is embedded with four pantoprazole sodium enteric-coated microtablets, calculated in 1000 tablets.
[0054] Pantoprazole sodium tablet core contains the following components:
[0055]
[0056] The isolation layer includes the following components:
[0057]
[0058] Add water and be mixed with 8% solid content solution, and isolation layer accounts for 15% of the mass of pantoprazole sodium enteric-coated microtablet;
[0059] The enteric layer includes the following components:
[0060]
[0061] Water is added to prepare a solution with a solid content of 20%, and the enteric layer accounts for 20% of the mass of the pantoprazole sodium enteric-coated microtablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com