Method for preparing lactic acid by using alkaline-earth metal modified Sn-beta catalyst
An alkaline earth metal and catalyst technology, applied in the field of lactic acid production, can solve the problems of limited sources of monosaccharide and polysaccharide reactants, low cellulose hydrolysis efficiency, small contact area, etc., and achieve short preparation cycle, high atom utilization rate, and process easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of catalyst:
[0026] The preparation method of Sn-β-Ca is: mix 0.4g Sn-β catalyst with 20mL 1.0M Ca(NO 3 ) 2 The solution was subjected to ion exchange reaction at 353K, the reaction time was 12h, after centrifugation, the obtained solid was washed with deionized water several times, then the obtained solid was dried at 120°C for 12h, and then calcined at 773K in a muffle furnace for 5h, and the obtained catalyst was recorded as It is Sn-β-Ca.
[0027] (2) Preparation of lactic acid:
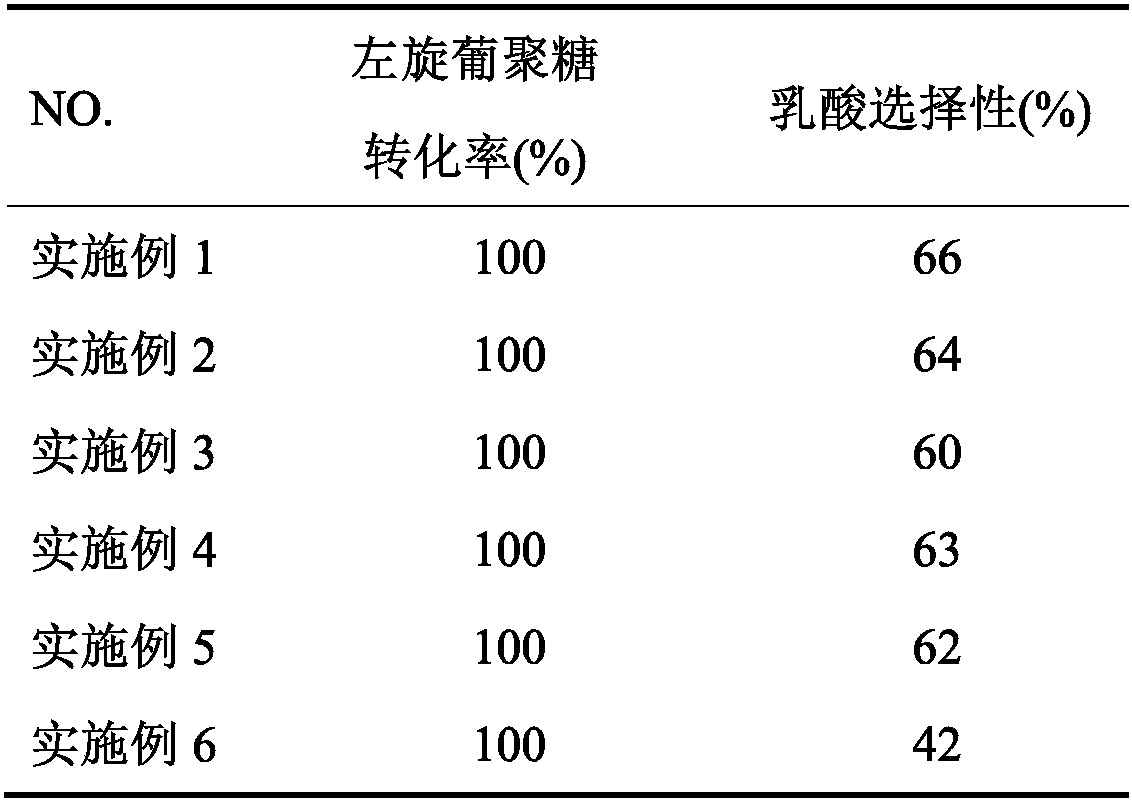
[0028] With 0.05g levoglucosan as substrate in reactor, 20mL deionized water is solvent, add the catalyst Sn-beta-Ca prepared in 0.2g step (1), add the lactic acid of 0.2mmol as B acid; Reaction conditions For: the reaction pressure is 2MPa N2, the reaction temperature is 190°C, and the reaction time is 120min. After the reaction finishes, carry out the productive rate analysis of lactic acid with high performance liquid chromatography, and the productive rate result ...
Embodiment 2
[0030] The preparation step of catalyst is similar to embodiment 1, and difference is: Ca(NO 3 ) 2 The solution was changed to Mg(NO 3 ) 2 Solution, the gained catalyzer is recorded as Sn-β-Mg; The preparation of lactic acid is similar to embodiment 1, and difference is: the catalyst Sn-β-Be is replaced by catalyst Sn-β-Mg; All the other conditions are the same as the productive rate of lactic acid The results are shown in Table 1.
Embodiment 3
[0032] The preparation step of catalyst is similar to embodiment 1, and difference is: Ca(NO 3 ) 2 The solution was changed to Be(NO 3 ) 2 Solution, the gained catalyzer is recorded as Sn-β-Be; The preparation of lactic acid is similar to embodiment 1, and difference is: change catalyzer Sn-β-Ca into catalyzer Sn-β-Be; All the other conditions are the same productive rate of lactic acid The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com