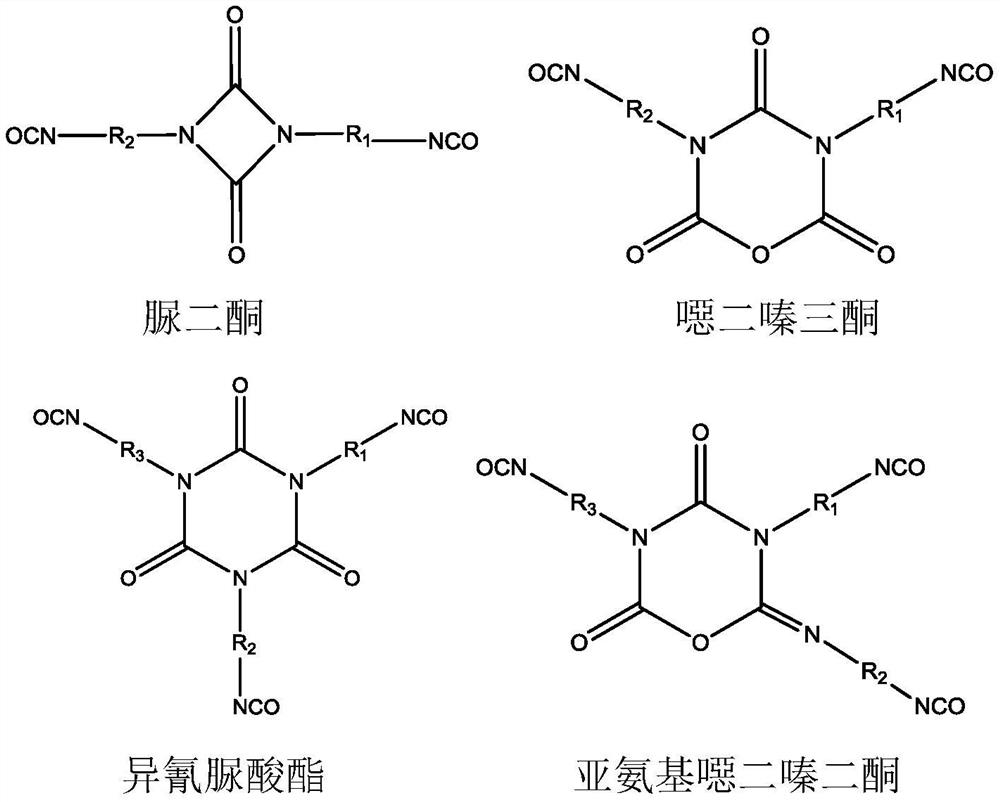
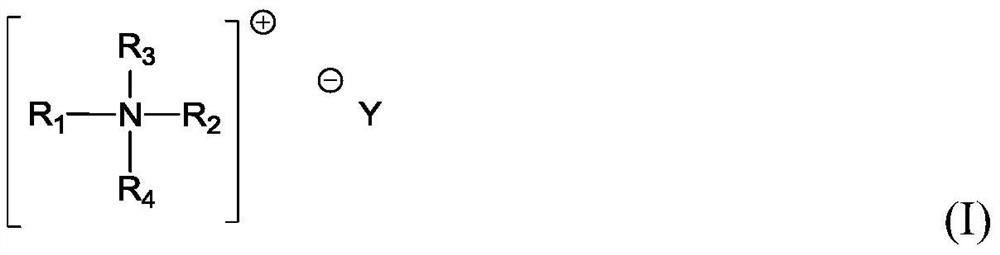A kind of polyisocyanate composition and preparation method thereof
A polyisocyanate and isocyanurate technology, applied in the field of isocyanates, can solve problems such as affecting product use, precipitation, and turbidity increase, and achieve the effects of reducing dosage, improving low temperature stability, and slowing decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] 1000 g of HDI was placed in a round bottom flask equipped with a reflux condenser, a stirrer, a thermometer and a nitrogen inlet, wherein the bromine content of HDI was 10 ppm.
[0076] The above reaction system was heated to 80°C, and then a n-hexanol solution of 50wt% tetrabutylammonium difluoride was added dropwise as a catalyst (100ppm of acetone was added to the catalyst solution in advance), stirring continuously during the reaction process, and the temperature was raised during the reaction. The rate of catalyst addition controls the reaction temperature between 80-90°C. When the monomer conversion rate of the reaction liquid system is 30%, immediately add dibutyl phosphate equal to the molar amount of the catalyst to terminate, and the reaction time is 5 hours. Obtain the reaction solution.
[0077] Use a thin film evaporator to evaporate and remove unreacted monomers under the conditions of temperature 130° C. and absolute pressure 100 Pa, so that the content i...
Embodiment 2
[0081] 1000 g of HDI was placed in a round bottom flask equipped with a reflux condenser, a stirrer, a thermometer and a nitrogen inlet, wherein the HDI had a bromine content of 30 ppm.
[0082] The above reaction system was heated to 60°C, and then a n-octanol solution of 50wt% benzyltrimethylammonium fluoride as a catalyst was added dropwise (500ppm of acetone was added to the catalyst solution in advance), stirring continuously during the process, and the temperature rose during the reaction. Control the reaction temperature between 60-70°C by controlling the catalyst addition rate, when the monomer conversion rate of the reaction liquid system is 20%, immediately add dibutyl phosphate in an equimolar amount to the catalyst to terminate, and the reaction time is 10 hours to obtain a reaction solution.
[0083] Use a thin film evaporator to evaporate and remove unreacted monomers under the conditions of temperature 130° C. and absolute pressure 100 Pa, so that the content is...
Embodiment 3
[0087] 1000 g of HDI was placed in a round bottom flask equipped with a reflux condenser, a stirrer, a thermometer and a nitrogen inlet, wherein the HDI had a bromine content of 30 ppm.
[0088] The above reaction system was heated to 70°C, and then the catalyst 50wt% tetramethylammonium fluoride in 2-ethyl-1,3-hexanediol solution was added dropwise (1000ppm of 2-butanone was added to the catalyst solution in advance), the process During the reaction process, the temperature rises, and the reaction temperature is controlled between 70-80°C by controlling the catalyst addition rate. When the monomer conversion rate of the reaction liquid system is 15%, immediately add dibutyl phosphate in an equimolar amount to the catalyst. The termination was carried out, and the reaction time was 8 hours at this time to obtain a reaction liquid.
[0089] Use a thin film evaporator to evaporate and remove unreacted monomers under the conditions of temperature 130° C. and absolute pressure 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com