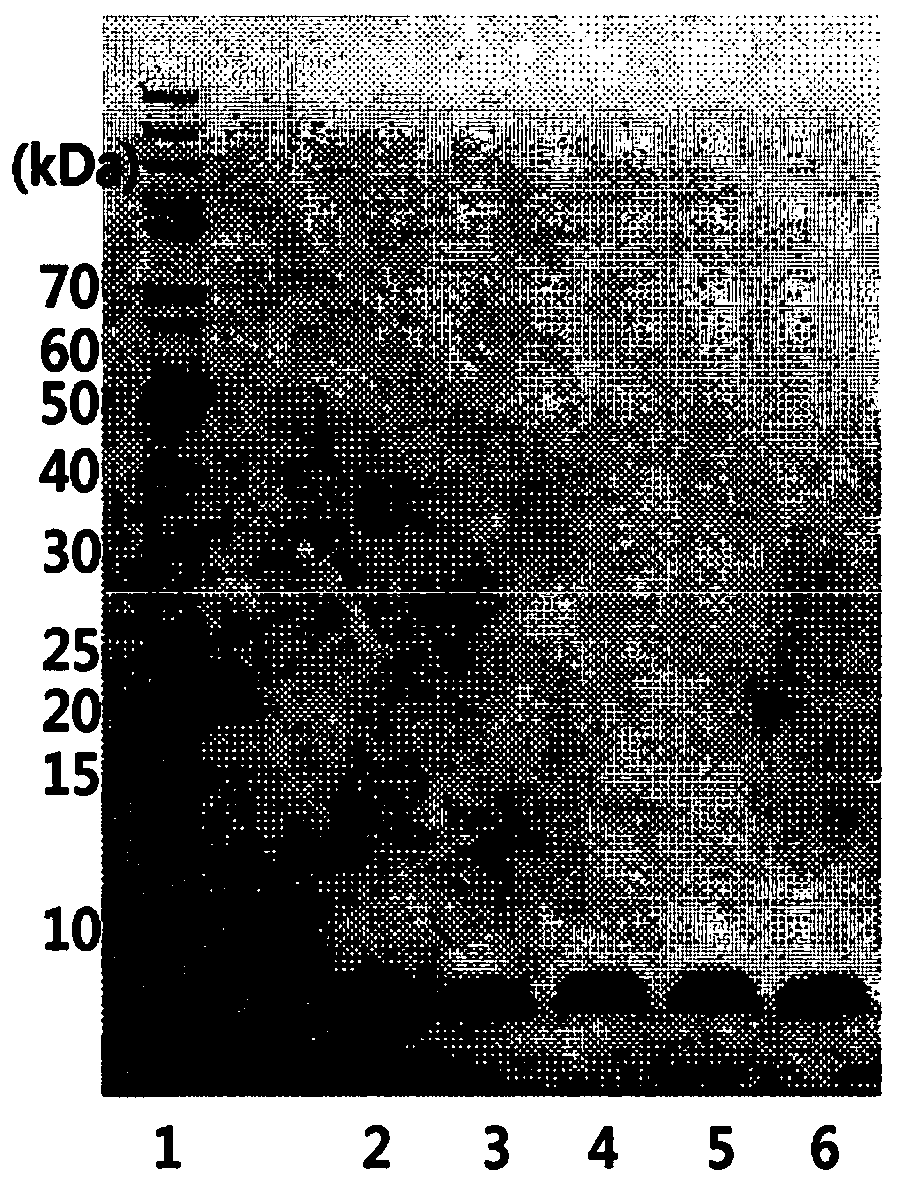
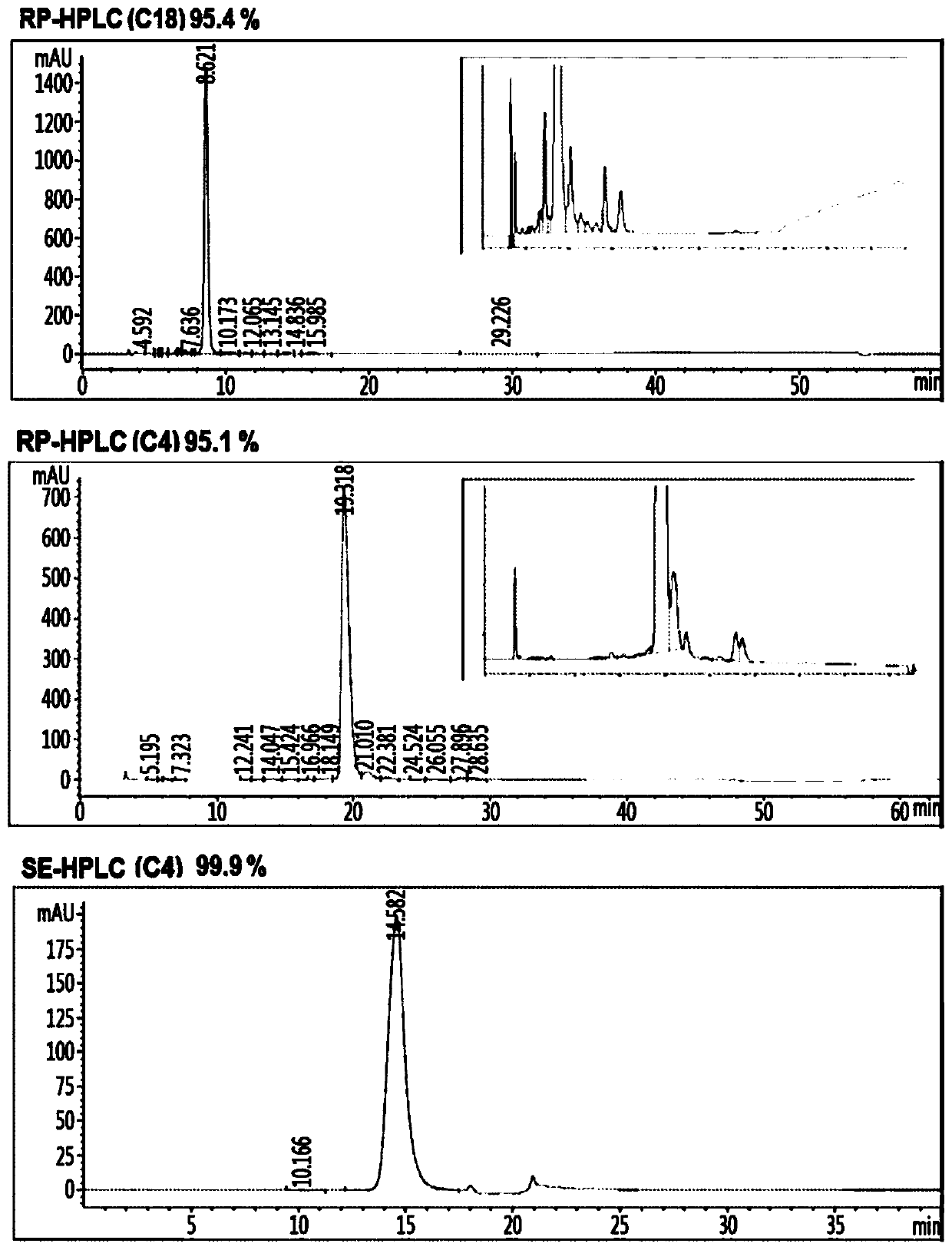
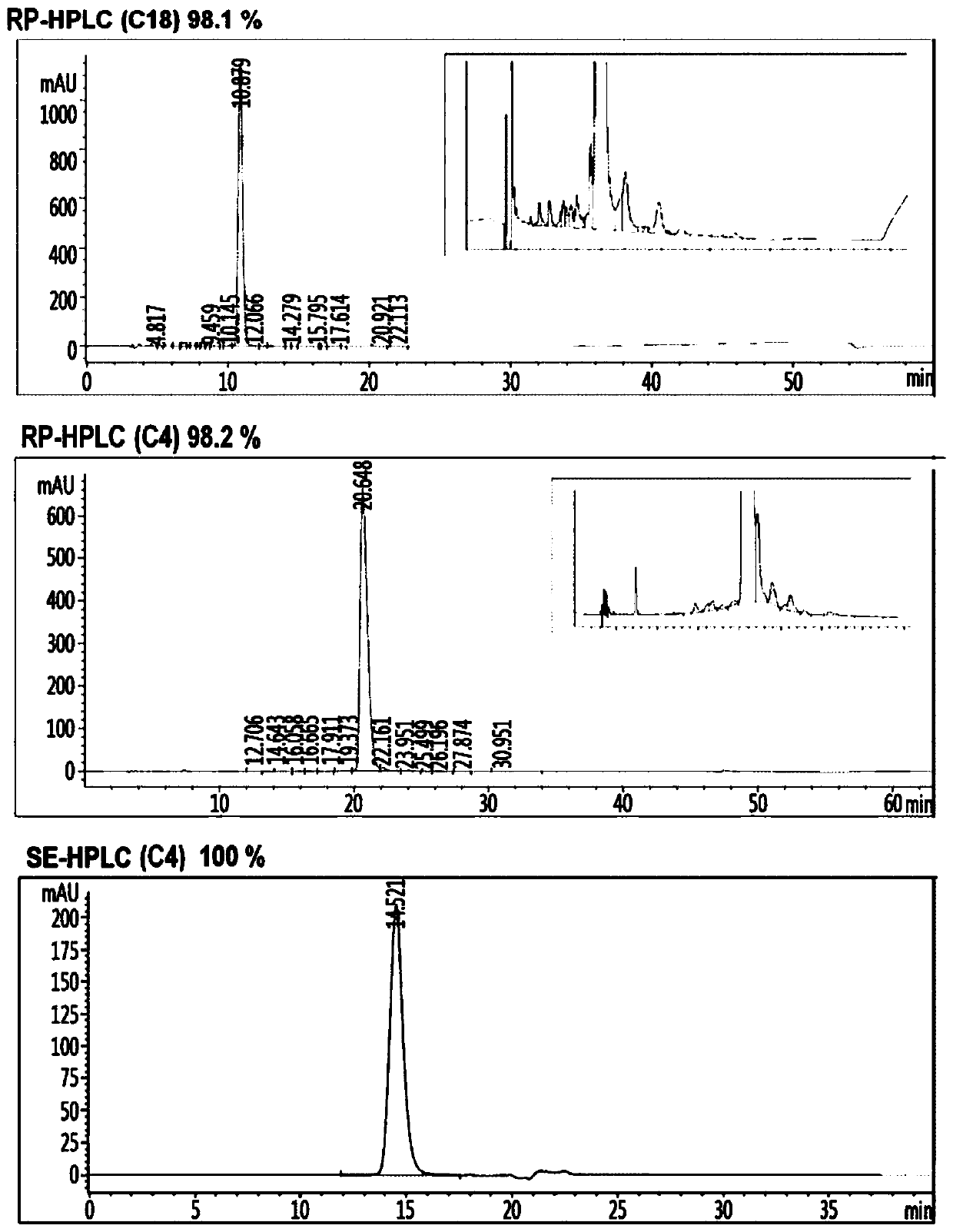
Insulin analog complex with reduced affinity for insulin receptor and use thereof
A technology for insulin and conjugates, applied in the field of conjugates of insulin analogs, can solve the problems of no specificity, reduced activity, no change in binding affinity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0210] Insulin analogs can be readily prepared by those skilled in the art using peptide preparation methods known in the art. For example, insulin analogs can be produced by a method comprising the steps of:
[0211] a) expressing an insulin analog by culturing a transformant comprising a nucleic acid encoding the insulin analog; and
[0212] b) Isolation and purification of the expressed insulin analog.
[0213] The medium used for culturing the transformant of the present invention can meet the requirements of host cell culture in an appropriate manner. The carbon source contained in the medium used for host cell growth can be appropriately selected by those skilled in the art according to the transformants prepared therefrom, and appropriate culture conditions can be selected to control the period and amount of culture.
[0214] Examples of sugar sources used may include sugars and carbohydrates such as glucose, sucrose, lactose, fructose, maltose, starch and cellulose; ...
Embodiment 1
[0327] Embodiment 1: Preparation of single-chain insulin analog expression vector
[0328] For the preparation of insulin analogues with a single modified amino acid in the A or B chains, respectively, using the native insulin expression vector in possession as a template, forward and reverse oligonucleotides were synthesized (Table 2) and then PCR amplified analogs for each gene.
[0329] Amino acid sequences and analog names modified in the A chain or B chain are shown in Table 1 below. In Table 1, analog 1 represents the analog in which the 14th amino acid (i.e. tyrosine, Y) of the A chain is replaced by histidine (H), and analog 6 represents the 16th amino acid in the B chain ( That is, an analogue in which tyrosine, Y) is replaced by glutamic acid (E).
[0330] [Table 1]
[0331]
[0332]
[0333] Primers used for insulin analog amplification are shown in Table 2 below.
[0334] [Table 2]
[0335]
[0336]
[0337] Eighteen repeated cycles of PCR reacti...
Embodiment 2
[0347] Example 2: Expression of Recombinant Insulin Analog Fusion Peptides
[0348] Expression of recombinant insulin analogues was performed under the control of the T7 promoter. Escherichia coli BL21-DE3 (Escherichia coli B F-dcm ompT hsdS(rB-mB-)galλDE3; Novagen) was transformed with each recombinant insulin analog expression vector. Transformations were performed according to the recommended protocol (Novagen). A single colony transformed with each recombinant expression vector was collected, inoculated into 2X Luria broth (LB) containing ampicillin (50 μg / mL), and incubated at 37°C for 15 hours. Mix the recombinant strain culture broth and 2X LB medium containing 30% glycerol at a ratio of 1:1 (v / v), dispense 1 mL of each mixture into cryovials, and store at -140 °C, which is used Used as a cell stock solution for the production of recombinant fusion proteins.
[0349] For expression of recombinant insulin analogues, one vial of each cell stock was thawed and inocula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com