RPA primers, probe sets and kit for detecting respiratory viruses
A virus detection and respiratory technology, which is applied in the direction of DNA/RNA fragments, recombinant DNA technology, microbial measurement/inspection, etc., can solve the problems of restricting the popularization and application of RPA technology, the difficulty of RPA primers, and the lack of good software analysis of RPA primers. Achieve good specificity, high sensitivity, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of RPA primers and probe sets for respiratory virus detection
[0036] 1. Alignment of conserved sequences and construction of cloning vectors
[0037] (1) Download the cDNA or DNA sequences encoding the same protein of the three viruses from the NCBI gene bank (download at least 40 sequencing sequences from different regions in the past 5 years for each virus);
[0038] (2) Use DNAman software to compare sequences, find out relatively conserved sequences, and design and optimize primers and probes.
[0039] The determined conserved sequence is as follows:
[0040]
[0041]
[0042] 2. Design of primers and probes
[0043] RPA primer design method:
[0044] (1) Select 32-35 bases from the single strand of the conserved sequence of the virus compared as the upstream and downstream primers.
[0045] (2) Control the base length between the two primers between 200-400bp.
[0046] (3) The dNTP at the 5' end avoids guanine.
[0047] (4) Control the GC ...
Embodiment 2
[0069] Composition of kits for detection of respiratory viruses
[0070] Including primers, probe sets and detection reagents in Example 1.
[0071] The detection reagent preferably includes RPA buffer, dNTP, recombinase, polymerase, magnesium acetate and water, wherein the RPA buffer, dNTP, recombinase and polymerase are derived from the RPA-exo kit of Twist Company.
[0072] The method of use of the test kit:
[0073] (1) Dissolve the lyophilized tube containing dNTP, recombinant enzyme and polymerase dry powder with RPA buffer.
[0074] (2) Add upstream primers, downstream primers, templates and water for RPA amplification, and the total volume of each sample is 47.5 μL. The loading reagents and volumes are shown in Table 1.
[0075] (3) Amplification starts after adding 2.5 μL of magnesium acetate solution, shake and briefly centrifuge.
[0076] (4) Add the centrifuged solution system into a constant temperature fluorescence detector (GEN3-02-P1), set the temperature a...
Embodiment 3
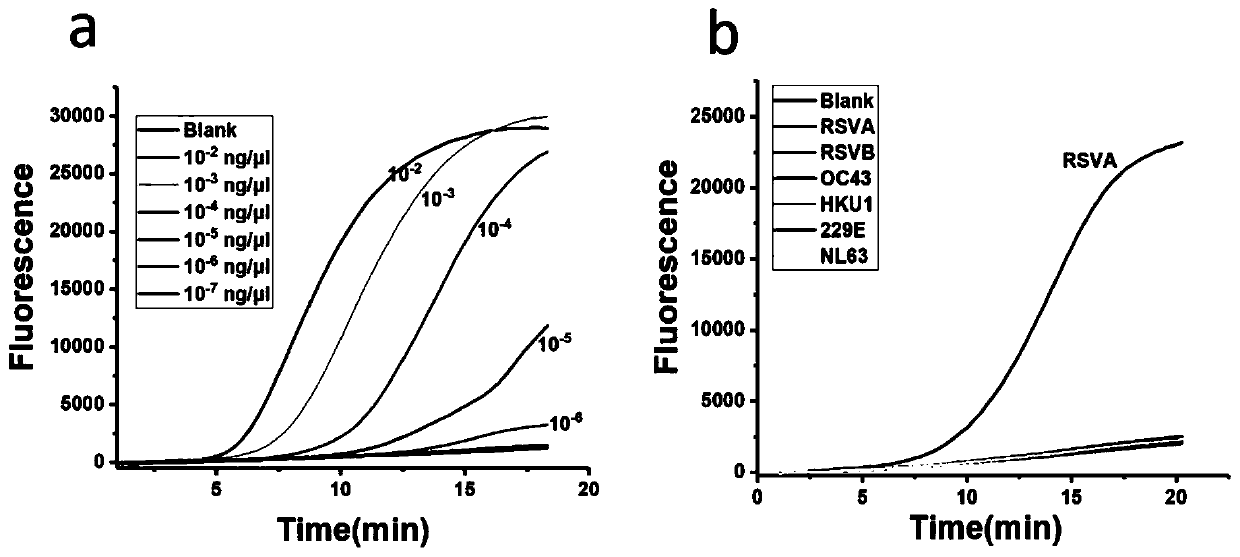
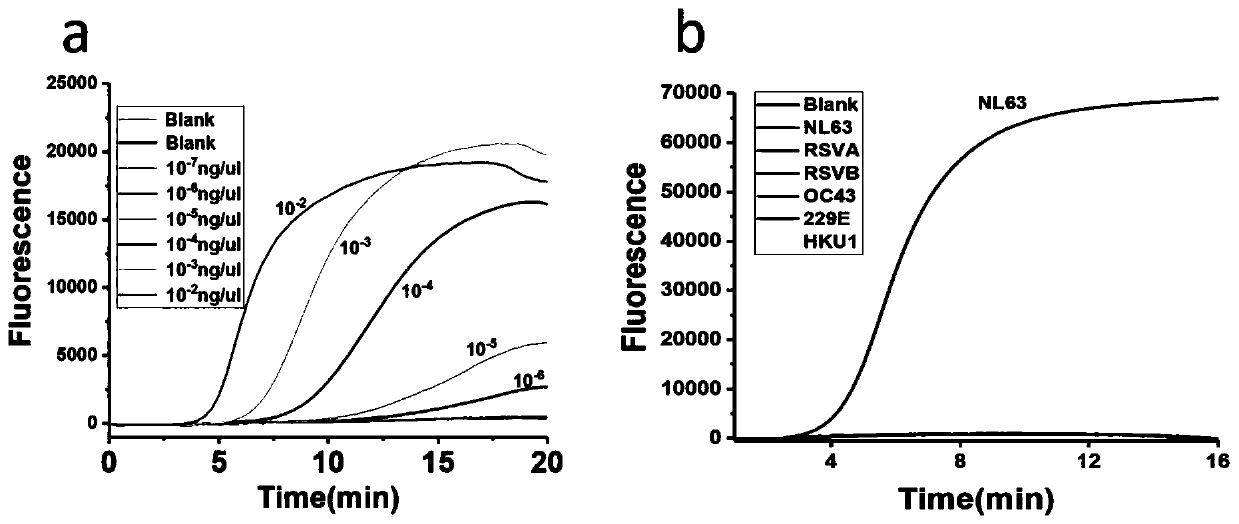
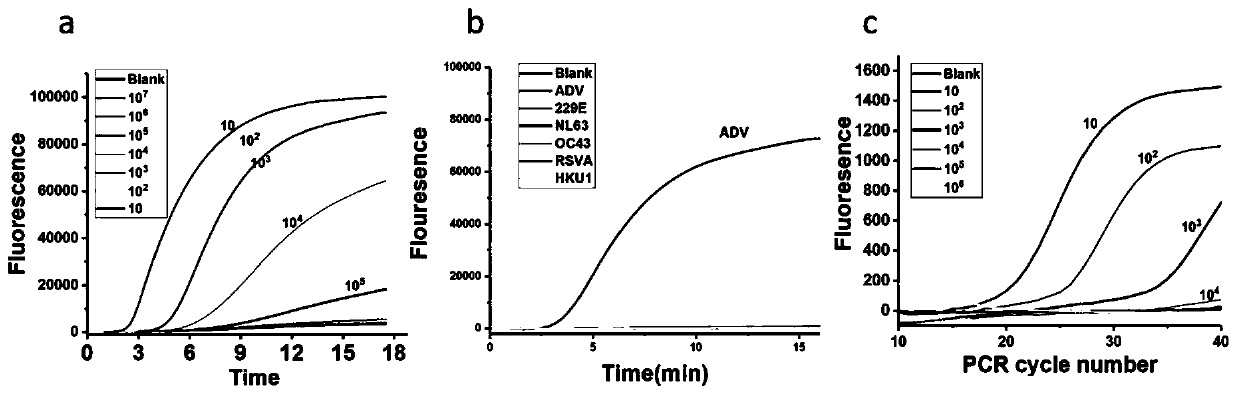
[0098] Comparison between the detection results of the primers, probe sets and kits provided by the invention for respiratory viruses and the detection results of the fluorescent quantitative PCR method.
[0099] Taking adenovirus ADV as an example, the same sample was detected by fluorescent quantitative PCR and the kit method provided by the present invention, and the results were compared.
[0100] Real-time quantitative PCR detection of adenovirus ADV:
[0101] The upstream primer for fluorescent quantitative PCR detection: gaggagccagatattgatatggaatt (SE Q ID No.13);
[0102] Downstream primer: aattgacattttccgtgtaaagca (SEQ ID No.14);
[0103] Fluorescent probe: FAM-aagctgctgacgctttttcgcctga-BHQ1 (SEQ ID No. 15) for detection.
[0104] For the steps of the method for detecting adenovirus ADV with the kit provided by the present invention, refer to Example 2.
[0105] As can be seen from the results, through the continuous increase of the number of cycles, the detection ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com