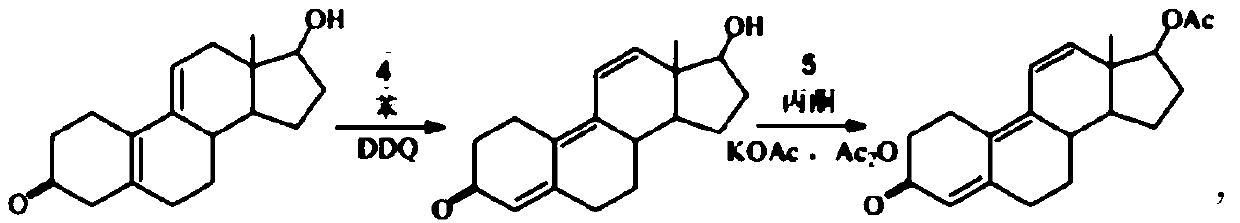
Method for preparing trenbolone acetate
A technology of trenbolone acetate and acid solution, which is applied in the field of preparation and processing of hormone drugs, can solve the problems of no obvious improvement in total yield, difficulty in purification, serious environmental pollution, etc., and achieves high production cost and operability, high raw material Inexpensive and easy to obtain, the effect of simple reaction control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Example 1 reduction reaction: 3-ethylenedioxyestradiol-Δ 5,10 ,Δ 9,11 - Preparation of Dien-17-ols 1
[0040] Add 10 grams of 3-ethylenedioxyeststrone-Δ to the reaction flask 5,10 ,Δ 9,11 - Diene-17-one (1), 80 ml of isopropanol, 5 g of sodium borohydride, heated to 60 ° C and stirred for reaction, after the reaction was completed, cooled to 10 ° C, water analysis, filtered to precipitate solids, and dried to obtain 9.2 g of 3-ethylenedioxyestr-Δ 5,10 ,Δ 9,11 - Dien-17-ol (2).
example 2
[0041] Example 2 reduction reaction: 3-ethylenedioxyestradiol-Δ 5,10 ,Δ 9,11 - Preparation of Dien-17-ol 2
[0042] Add 10 grams of 3-ethylenedioxyeststrone-Δ to the reaction flask 5,10 ,Δ 9,11- Diene-17-one (1), 100 ml of ethanol, 10 g of potassium borohydride, controlled temperature 0 ° C, stirred and reacted, after the reaction was completed, cooled to -5 ° C, water analysis, filtered to precipitate solids, dried to obtain 9.1 gram 3-ethylenedioxyestr-Δ 5,10 ,Δ 9,11 - Dien-17-ol (2).
example 3
[0043] Example 3 reduction reaction: 3-ethylenedioxyestradiol-Δ 5,10 ,Δ 9,11 - Preparation of Dien-17-ol 3
[0044] Add 10 grams of 3-ethylenedioxyeststrone-Δ to the reaction flask 5,10 ,Δ 9,11 - Dien-17-one (1), 80 ml of methanol, 5 g of potassium borohydride, heated to 40 ° C and stirred for reaction, after the reaction was completed, cooled to 6 ° C, water analysis, filtered to precipitate solid, and dried to obtain 9.0 g 3-ethylenedioxyestr-Δ 5,10 ,Δ 9,11 - Dien-17-ol (2).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com