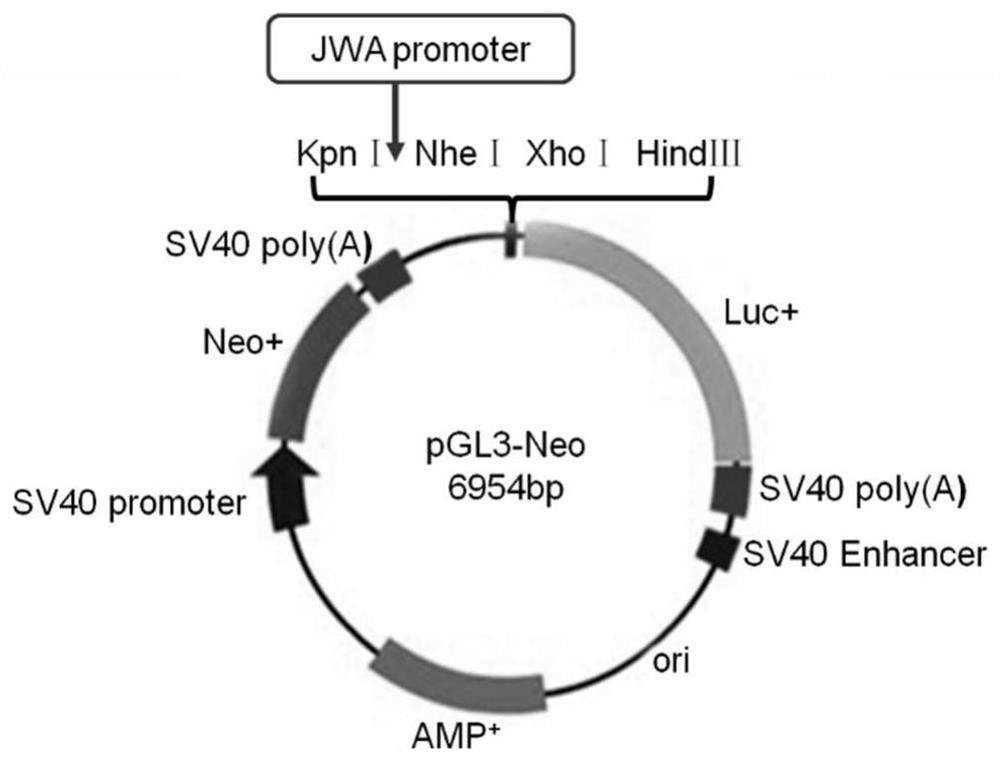
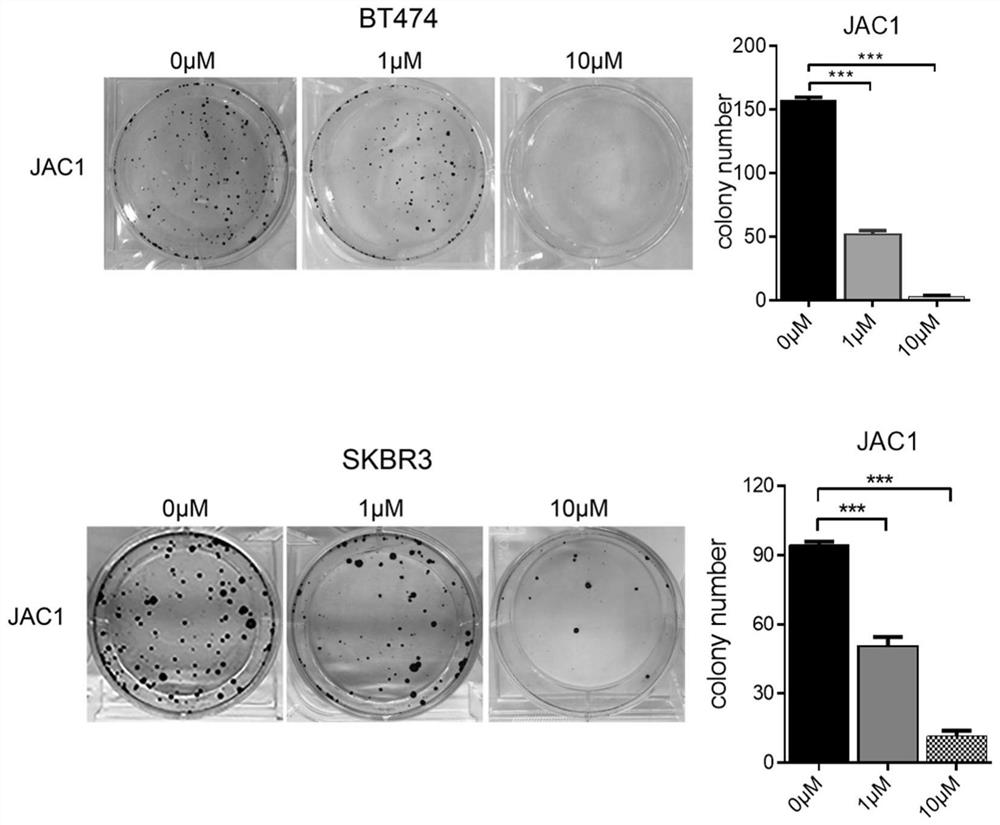
An anti-tumor compound based on activating jwa gene and degrading her2, its preparation method and its application
A compound and anti-tumor technology, applied in the field of anti-tumor drugs, can solve problems such as ineffectiveness, achieve broad application prospects, and significantly treat tumors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of 4-fluoro-N-(4-methoxybenzo[d]thiazol-2-yl)benzo[d]thiazol-2-amine:
[0060] In a 50mL dry eggplant-shaped bottle equipped with a spherical reflux condenser and a magnet, add 2-chloro-4-fluorobenzo[d]thiazole (1g, 5.3mmol), 4-methoxybenzo[d]thiazole -2-amine (0.96g, 5.3mmol), cuprous iodide (0.31g, 0.3mmol), potassium carbonate (1.47g, 10.6mmol), dimethyl sulfoxide (20ml), heated to 100°C, reaction 6 Hour. After the reaction, cool to room temperature, add water (60ml), ethyl acetate (30mL), extract 3 times, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and evaporate the solvent under reduced pressure to obtain a black solid, crude Product silica gel powder sand making, silica gel chromatographic column purification [eluent: V PE :V EA =5:1] to obtain a black solid, which was slurried several times with ethyl acetate or methanol to obtain a white solid compound (0.7 g, yield: 39%).
[0061] 1 H NMR (400MHz, DMSO...
Embodiment 2
[0064] Preparation of 4,7-difluoro-N-(4-methoxybenzo[d]thiazol-2-yl)benzo[d]thiazol-2-amine:
[0065] With reference to the method shown in Example 1, wherein "2-chloro-4-fluorobenzo[d]thiazole" was replaced by "2-chloro-4,7-difluorobenzo[d]thiazole", an off-white solid (1.1 g, yield: 57%).
[0066] 1 H NMR (400MHz, DMSO-d 6 ) δ 7.73 (d, J = 5.4Hz, 1H), 7.28-7.17 (m, 3H), 7.02 (d, J = 8.0Hz, 1H), 3.92 (s, 3H).
[0067] ESI-MS m / z:350.1{[M+H] +}.
Embodiment 3
[0069] Preparation of 4,6-difluoro-N-(4-methoxybenzo[d]thiazol-2-yl)benzo[d]thiazol-2-amine:
[0070] With reference to the method shown in Example 1, wherein "2-chloro-4-fluorobenzo[d]thiazole" is replaced by "2-chloro-4,6-difluorobenzo[d]thiazole", an off-white solid (0.8 g, yield: 45%).
[0071] 1H NMR (400MHz, DMSO-d 6 )δ7.65(d, J=5.8Hz, 1H), 7.35(d, J=7.9Hz, 1H), 7.28-7.17(m, 2H), 7.03(d, J=8.0Hz, 1H), 3.92( s, 3H).
[0072] ESI-MS m / z:350.1{[M+H] +}.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com