Sodium alginate diester anti-tumor nano preparation and preparation method
A technology of sodium alginate diester and nano-suspension, which is applied in the direction of antineoplastic drugs, pharmaceutical formulas, medical preparations of non-active ingredients, etc. It can solve the problems of nano-suspension instability and achieve significant crystal inhibition performance , Overcome the effect of low drug loading, strong dispersion emulsification and crystal inhibition performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
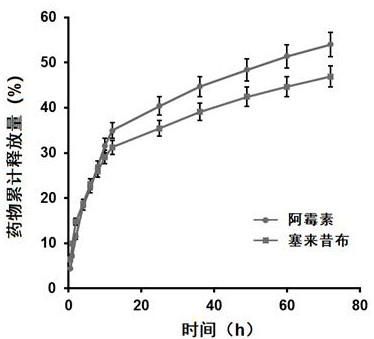
[0055] 1. Preparation of doxorubicin / celecoxib sodium alginate diester nanosuspension
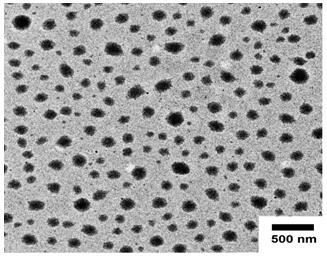
[0056] Weigh 25 mg of sodium alginate diester (School of Medicine, Ocean University of China) and 5 mg of polyvinylpyrrolidone (PVPK30, purchased from Sigma-Aldrich), dissolve them in 15 mL of distilled water, and stir to fully dissolve them. Doxorubicin hydrochloride (purchased from Dalian Meilun Biotechnology Co., Ltd.) was dissolved in dimethyl sulfoxide at a concentration of 8 mg / mL, and 3 times the molar equivalent of triethylamine was added. Stir at room temperature for 12 h in the dark for desalination; dissolve celecoxib (Dalian Meilun Biotechnology Co., Ltd.) in dimethyl sulfoxide at a concentration of 80 mg / mL; Equal volumes of the coxib solution were mixed and stirred for 24 h in the dark. Then 2 mL of the drug solution was slowly added dropwise to the above-mentioned sodium alginate diester solution, stirring was continued at room temperature for 30 min, and the rotation speed w...
Embodiment 2
[0088] 1. Preparation of doxorubicin / celecoxib sodium alginate diester nanopowder injection
[0089] Add about 2% mannose to the doxorubicin / celecoxib sodium alginate nanosuspension prepared in Example 1, and then freeze-dry to obtain a reddish-brown flocculent powder, which is its Nano powder injection.
[0090] 2. Determination of drug content in doxorubicin / celecoxib diester sodium alginate nanopowder injection
[0091] The same HPLC method as in Example 1 was used to detect the contents of doxorubicin and celecoxib in the nanopowder injection. Sample processing and determination of drug content: when detecting the content of doxorubicin, accurately weigh 5 mg of nanopowder injection and disperse it in 5 mL of 0.1mol / L hydrochloric acid solution, vortex for 3 h and then filter, and dilute the filtrate by an appropriate multiple according to In Example 1, the concentration of doxorubicin was determined by sample injection under high performance liquid chromatography condi...
Embodiment 3
[0096] 1. Preparation of norcantharidin nanosuspension
[0097] Weigh 25 mg of sodium alginate diester and 5 mg of polyvinylpyrrolidone, dissolve them in 15 mL of distilled water, stir to dissolve them fully; weigh 30 mg of norcantharidin (purchased from Dalian Meilun Biotechnology Co., Ltd.) In 1.5 mL of acetone, slowly add dropwise to the above-mentioned sodium alginate diester solution, continue to stir at room temperature for 30 min, and control the rotation speed at 600-800 rpm to obtain a white milky suspension, and then rotary evaporate at room temperature to remove the acetone. Obtain norcantharidin nanosuspension.
[0098] 2. Determination of Drug Content in Norcantharidin Nanosuspension
[0099]The drug content of norcantharidin in the nanosuspension was detected by high performance liquid chromatography (HPLC). The chromatographic conditions are as follows: Waters C18 column (4.6×250 mm, 5 μm); mobile phase pH 3.1 phosphoric acid aqueous solution-methanol (70 / 30...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com