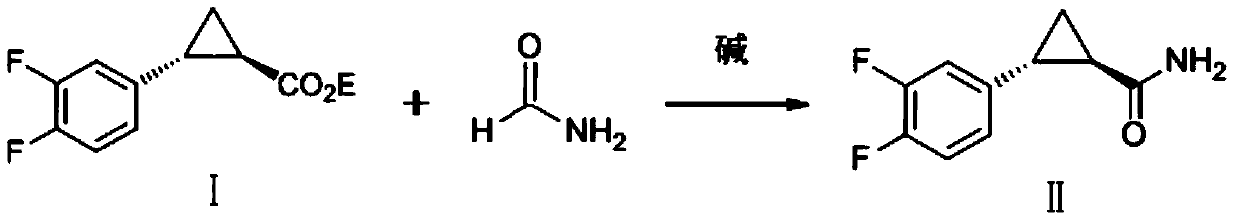
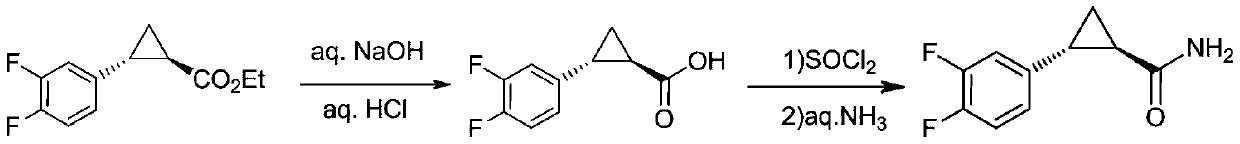
Preparation method of ticagrelor key intermediate aromatic cyclopropanamide
A technology of cyclopropaneamide and ticagrelor, which is applied in the field of preparation of aromatic cyclopropaneamide, a key intermediate of ticagrelor, can solve the problems of potential safety hazards in large-scale production, achieve easy purchase, save reaction steps, The effect of cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of the key intermediate aromatic cyclopropaneamide of ticagrelor, comprising the steps of:
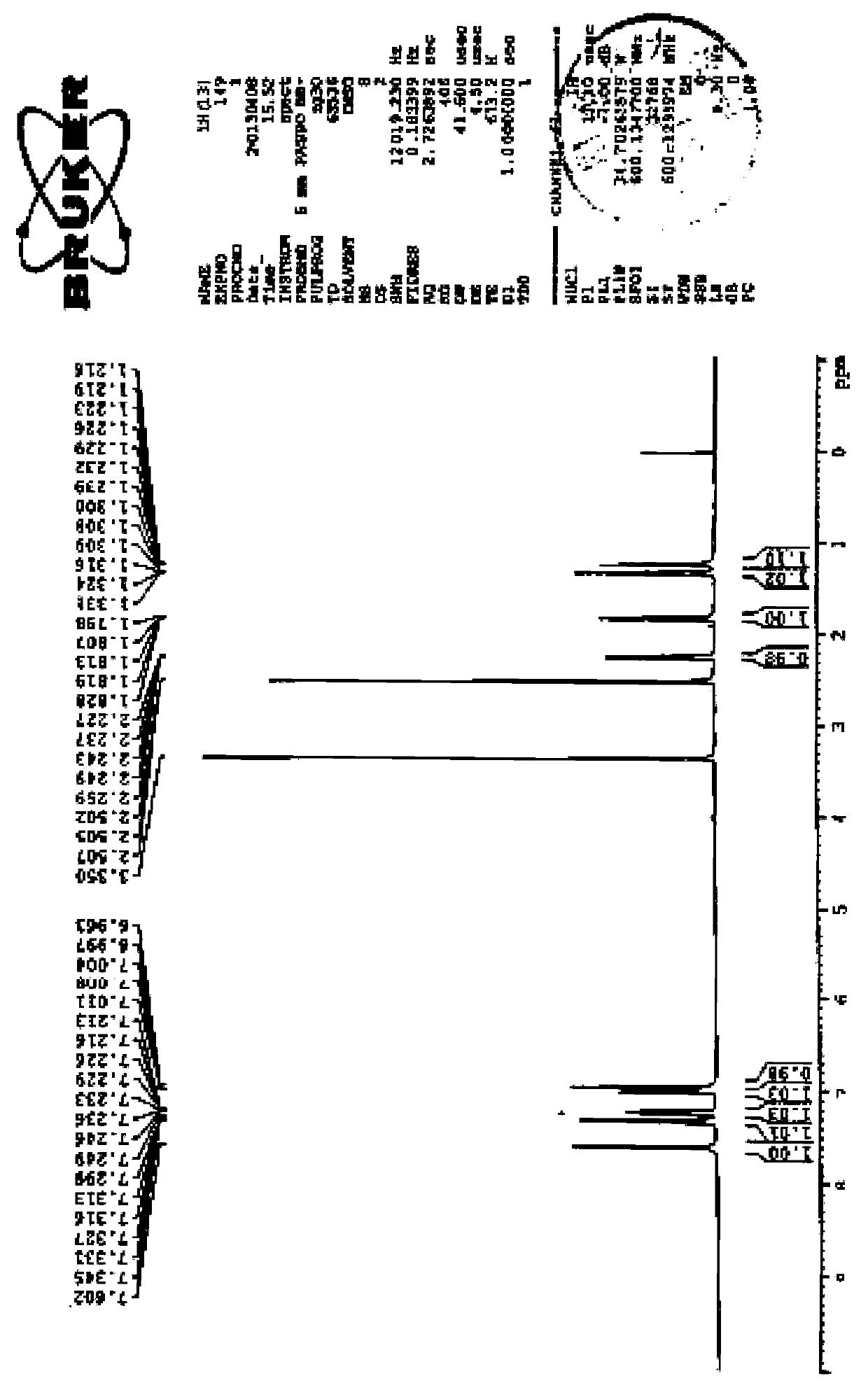
[0030] Dissolve ethyl (1R,2R)-2-(3,4-difluorophenyl)-1-cyclopropylcarboxylate (I) (20g, 88.496mmol, 1.0eq) in formamide (100ml ), added tetrabutylammonium bromide (1.43g, 4.425mmol, 0.05eq), added ethyl formate (3.278g, 44.248mmol, 0.5eq), and slowly added methanol solution (25wt%) of sodium methoxide under stirring ( 36.8g, 176.992mmol, 2.0eq), heat up to 55°C, keep warm for 2 hours, then seal the reaction bottle and draw negative pressure to -0.08MPa~-0.09MPa, keep warm for 5 hours, TLC (PE / EA, 5:1, V / V) Track the end of the reaction, cool the reaction solution to 0-5°C, add dropwise the prepared acetic acid (10.63g, 176.992mmol, 2.0eq) water (200ml) solution, stir for 30 minutes, filter, and rinse twice with 100ml of water , drained, put the wet product into the reaction flask, add dichloromethane (80ml), stir and heat up to reflux, stir for 1 hour, coo...
Embodiment 2
[0032] A preparation method of the key intermediate aromatic cyclopropaneamide of ticagrelor, comprising the steps of:
[0033]Dissolve ethyl (1R,2R)-2-(3,4-difluorophenyl)-1-cyclopropylcarboxylate (I) (40g, 176.992mmol, 1.0eq) in formamide (200ml ), add tetrabutylammonium bromide (2.86g, 8.85mmol, 0.05eq), add ethyl formate (6.56g, 88.496mmol, 0.5eq), slowly add ethanol solution (20wt%) of sodium ethylate under stirring (120.44g, 353.984mmol, 2.0eq), heat up to 60°C, keep warm for 2 hours, then seal the reaction bottle and draw negative pressure to -0.08MPa~-0.09MPa, keep warm for 5.5 hours, TLC (PE / EA, 5:1, V / V) Tracking the end of the reaction, cooling the reaction solution to 0-5°C, adding dropwise the prepared acetic acid (21.26g, 353.984mmol, 2.0eq) water (400ml) solution, stirring for 30 minutes, filtering, 150ml of water twice Wash, drain, put the wet product into a reaction bottle, add dichloromethane (160ml), stir and heat up to reflux, stir for 1 hour, cool down to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com