A kind of mesalazine colon-targeted controlled-release tablet and preparation method thereof
A technology of salazine colon and controlled-release tablets, which is applied in anti-inflammatory agents, pill delivery, pharmaceutical formulations, etc., can solve the problems of drug utilization to be improved, poor patient compliance, and unacceptable by patients, so as to delay disintegration time, Reduce loss, good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
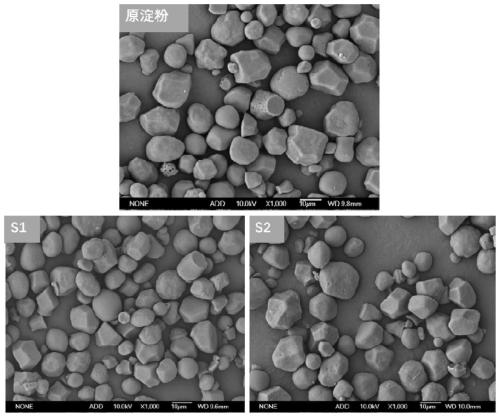
Image
Examples
Embodiment 1
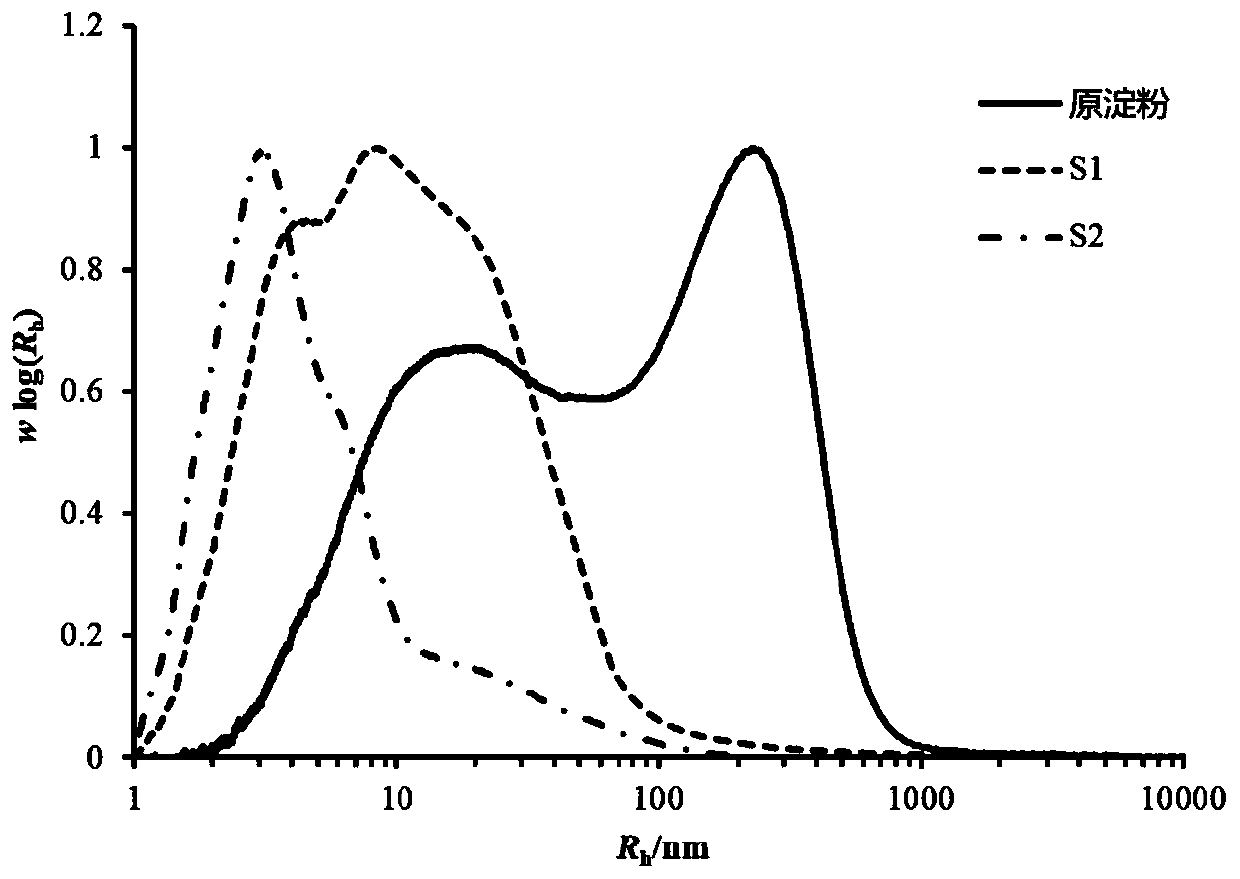
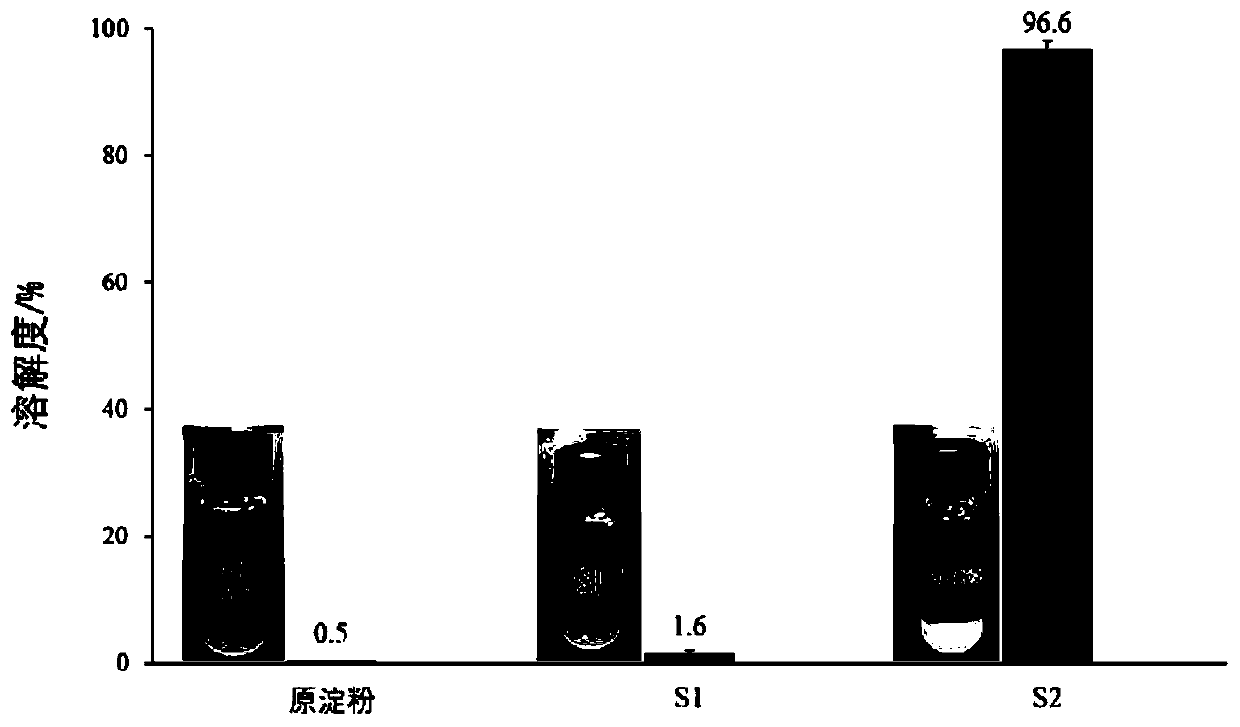
[0040] A mesalamine colon-targeted controlled-release tablet, composed of a core layer and a controlled-release layer outside the core layer, the core layer is composed of mesalamine and pyrodextrin as an auxiliary material, and the pyrodextrin as an auxiliary material The weight-average molecular size Rh is 1.8nm and the solubility is 96.6%. The controlled release layer is made of pyrodextrin with a weight-average molecular size Rh of 9.2nm and a solubility of 1.6%.
[0041] The weight ratio of mesalazine and auxiliary material pyrodextrin in the tablet core layer is 1:10.
[0042] The weight ratio of the excipient pyrodextrin in the tablet core layer to the pyrodextrin in the release-controlling layer is 1:2.
[0043] The preparation method of the mesalamine colon targeting controlled-release tablet comprises the steps:
[0044]The preparation process of the auxiliary material pyrodextrin in the S1 tablet core layer and the pyrodextrin in the controlled release layer compri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com