Acidic high-temperature-resistant recombinant cellulase and application thereof
A technology of cellulase and high temperature resistance, applied in the field of genetic engineering technology and biomass refining, can solve the problem of Avicel's inactivity, etc., and achieve the effect of broad substrate spectrum, easy expression, and stable acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Gene preparation of endoglucanase (Endo5), carbohydrate binding domain (CBM) and exoglucanase (Exo5).
[0042] The original endo5-cbm28, endo5, cbm3b-exo5 and exo5 genes in the prior art can be prepared artificially, or the total DNA of Caldicellulosiruptor saccharolyticus (purchased from the American Type Culture Collection) can be used as a template to obtain it by PCR.
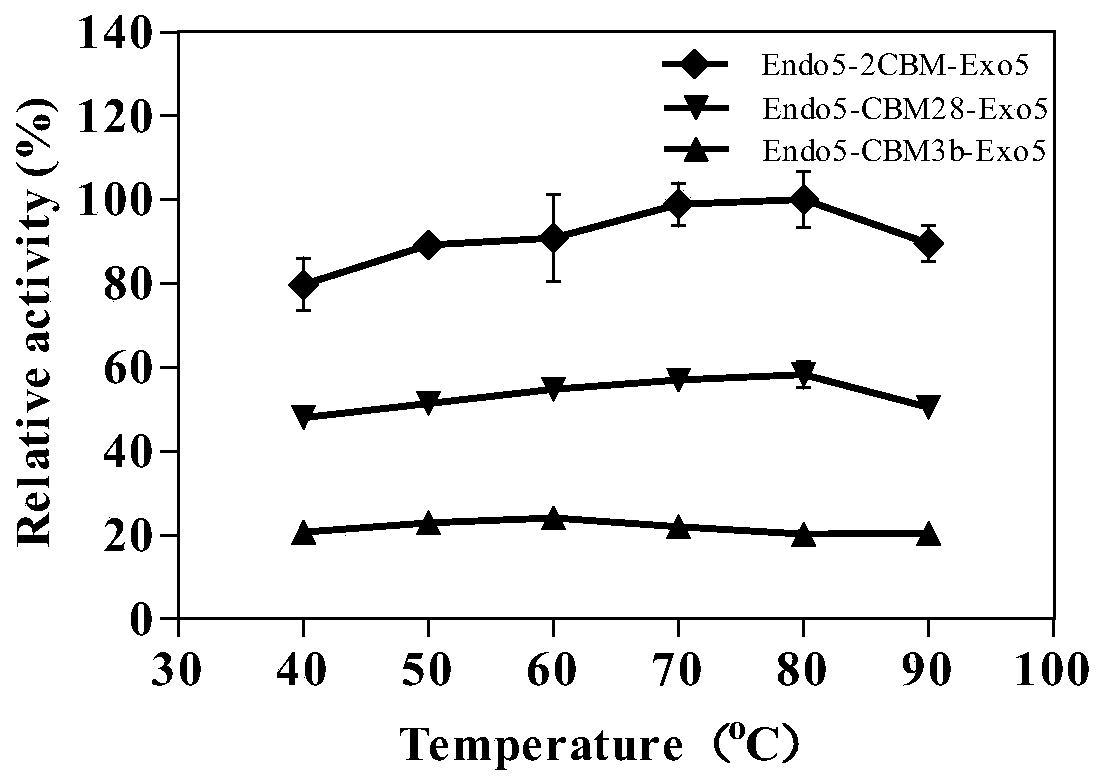
[0043] The brand-new endo5-cbm28, endo5, cbm3b-exo5 and exo5 genes in this example are based on the characteristics of enzymes Endo5-CBM28, Endo5, CBM3b-Exo5 and Exo5 binding to substrates, after sequence alignment, prediction and design of new sequences , and sent the new sequence to Shanghai Sangong for gene synthesis. The newly designed gene has better activity on substrates such as CMC-Na, filter paper, Avicel, and natural lignocellulose, and better enzymatic hydrolysis effect.
[0044] Using the new sequence synthesized from the whole gene as a template, the following new primer pairs (SEQ ID N...
Embodiment 2
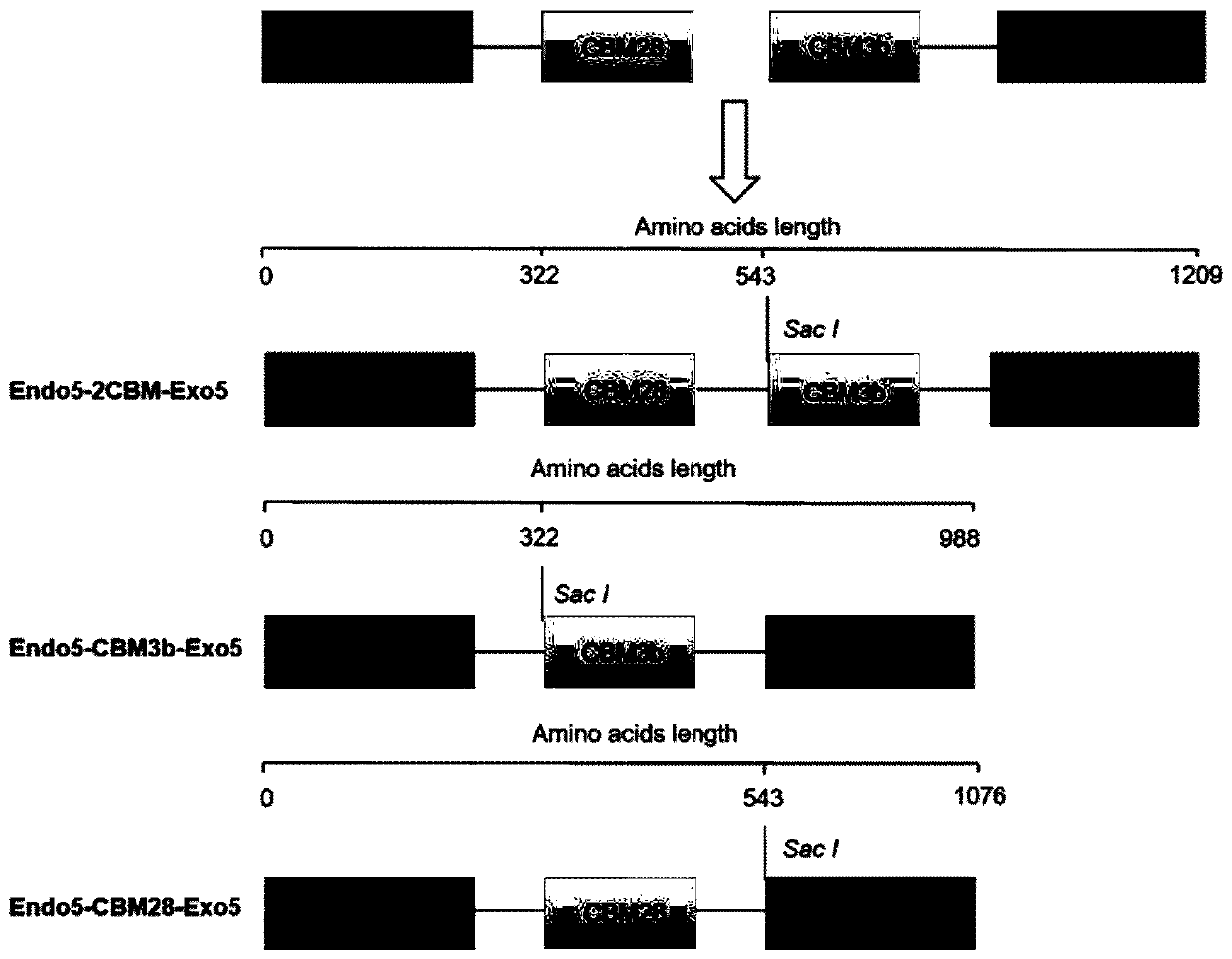
[0057] Construction and verification of recombinant clones and expression vectors pET28a-endo5-2cbm-exo5, pET28a-endo5-cbm3b-exo5 and pET28a-endo5-cbm28-exo5
[0058] The purified PCR products endo5-cbm28, endo5 (prepared in Example 1) and pET28a (Novagen) were double digested with NcoI and SacI, respectively, and the digested PCR fragments and large vector fragments were recovered by agarose gel electrophoresis. The target fragment and carrier recovered by tapping the gel were concentrated and added to 8 μL sterile water for resuspension. Add 1 μL 10×LigaseBuffer and 1 μL Ligase, and ligate overnight at 16°C. The ligation product was transformed into Escherichia coli (E.coliDH5a), and then spread on LB solid medium containing 50 μg / mL kanamycin (Kan), and cultured at 37°C for 13-15 hours.
[0059] Pick multiple single colonies from the transformation plate, and use the Shanghai Sangon Plasmid Extraction Kit to extract the plasmid. The extracted plasmids were verified by dou...
Embodiment 3
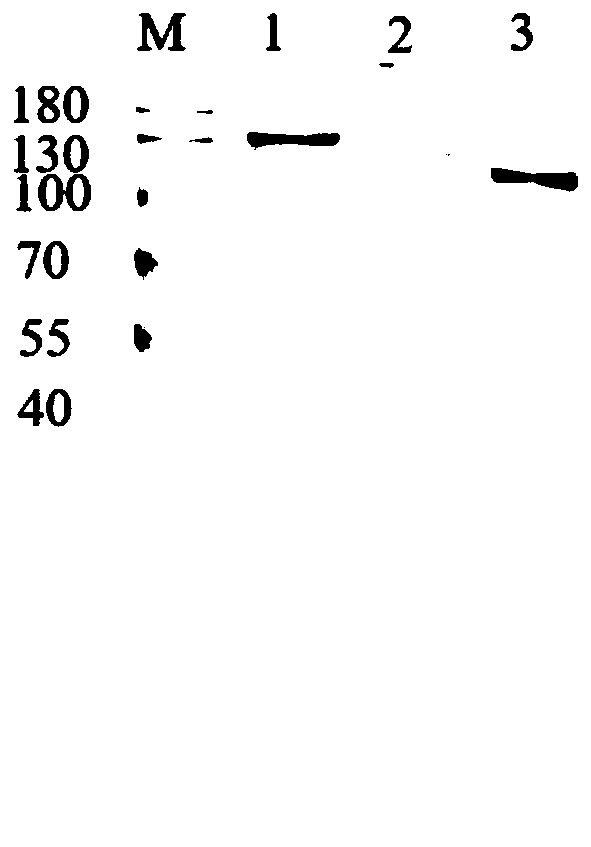
[0062] Expression and purification of recombinant cellulase Endo5-2CBM-Exo5, Endo5-CBM3b-Exo5 and Endo5-CBM28-Exo5.
[0063] Recombinant clones, expression vectors pET28a-endo5-2cbm-exo5, pET28a-endo5-cbm3b-exo5 and pET28a-endo5-cbm28-exo5 (prepared in Example 2) were chemically transformed into expression host E.coliBL21(DE3) (Novagen), Obtain the recombinant bacteria containing the recombinant plasmid. Positive recombinants were inoculated in 5 mL of Luria-Bertanibroth (LB) liquid medium containing 50 μg / mL Kan, cultured overnight at 37° C. and 200 rpm. Transfer 4 mL of bacterial liquid to 500 mL of LB liquid medium containing 50 μg / mL Kan, and cultivate the bacterial concentration (OD 600 ) to 0.6-0.8. Add IPTG with a final concentration of 0.5mM, and induce expression at 25°C and 200rpm for 12-14h. The culture solution was centrifuged in a high-speed refrigerated centrifuge at 4°C, 10,000 rpm for 8 minutes to collect the bacterial cells. Add 40 mL of sterile water to w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com