A kind of prodrug and its preparation method and application
A prodrug and reaction technology, used in pharmaceutical formulations, drug combinations, drug delivery, etc., can solve problems such as large toxic side effects, poor targeting, and poor water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] (1) Preparation of succinylated paclitaxel: Suspend succinic anhydride in dichloromethane, add paclitaxel, stir, add anhydrous pyridine, stir and react for 24-48 hours; after reaction, distill under reduced pressure, add water to wash, centrifuge to collect the precipitate, and vacuum dry. to obtain succinyl paclitaxel;
[0076] Wherein, the mass ratio of succinyl to paclitaxel is 1:1-1:10, further 1:3-1:5, and the amount of pyridine is 0.1-0.3% (v / v) of the reaction system.
[0077] (2) Preparation of hyaluronic acid-cystamine: dissolve hyaluronic acid in PBS solution with pH 7.4, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride in sequence Salt (EDC·HCl), N-hydroxysuccinimide (NHS), stir at room temperature to activate the carboxyl group of HA for a period of time, add cystamine, continue stirring to obtain a crude product, which is purified by dialysis and freeze-dried to obtain HA-cys polymerization thing;
[0078] Wherein, the feeding of hyaluronic a...
Embodiment 1
[0089] Example 1 Synthesis of paclitaxel-hyaluronic acid-chlorin e6 polymer
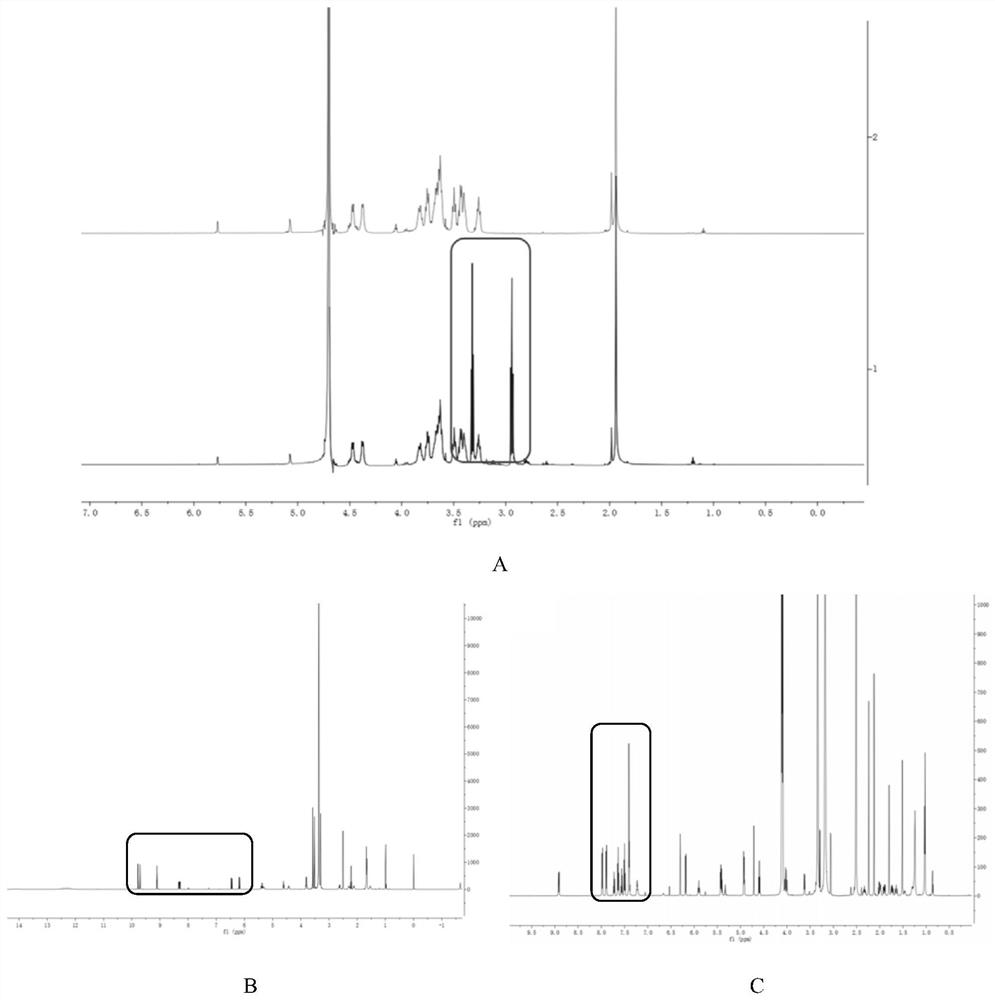
[0090] (1) Weigh 20mg of succinic anhydride, add 8mL of dichloromethane, magnetically stir to dissolve, add 60mg of PTX, magnetically stir to dissolve, add 60μL of anhydrous pyridine, and stir for 24 hours after the reaction system is clarified. Distill under reduced pressure, add 10 mL of water, stir for 15 min, collect the precipitate after centrifugation, wash the precipitate with 30 mL of water again, and dry in vacuo to obtain succinylated paclitaxel (compound of formula 1).
[0091] (2) Weigh 0.2g hyaluronic acid, dissolve it in 50mL PBS (pH=7.4), add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC· HCl) 78.2mg, N-hydroxysuccinimide (NHS) 47.0mg, stirred at room temperature to activate the carboxyl group of HA for 30min, added 1.12g of cystamine, and continued to stir for 24h to obtain a crude product, which was purified by water dialysis and freeze-dried to obtain hyaluronic...
Embodiment 2
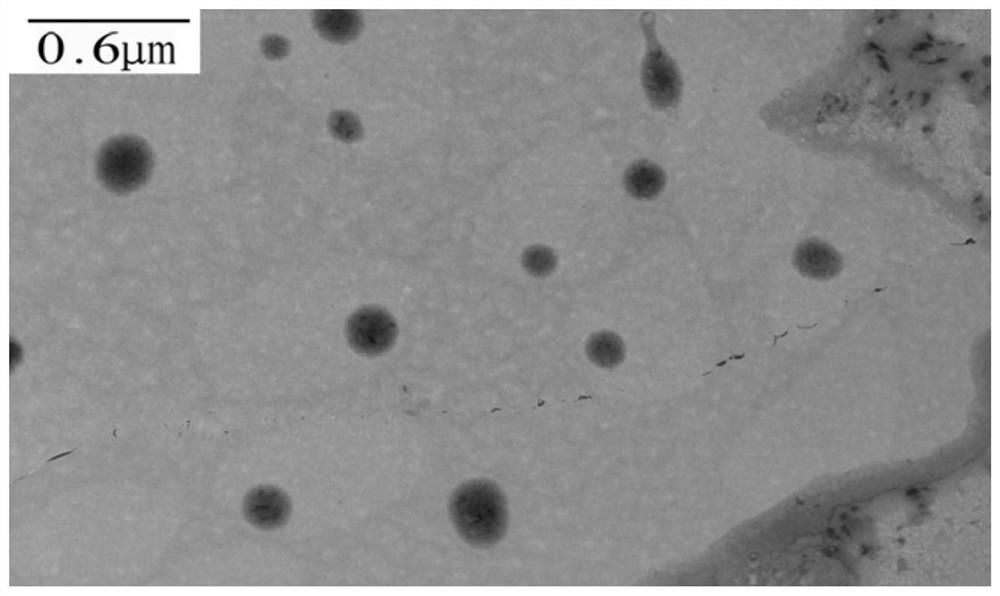
[0096] Weigh 5mg of the paclitaxel-hyaluronic acid-chlorin e6 polymer obtained in Example 1, dissolve it in 3mL of water, ultrasonicate the probe for 4min, the power is 40w, the ultrasonic pulse is turned on for 2s and turned off for 4s, and the obtained colloidal solution is passed through 0.45μm Microporous membrane to obtain green paclitaxel-hyaluronic acid-chlorin e6 polymer self-assembled nanoparticle solution, transmission electron microscope to investigate the shape of nanoparticles, see figure 2 .
[0097] To investigate the reduction sensitivity of self-assembled nanoparticles, incubate nanoparticles with high concentration (20mM) dithiothreitol (DTT) for 4h, compare the particle size and particle size distribution in DTT environment and water, see Figure 3-4 , the result is as follows:
[0098]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com