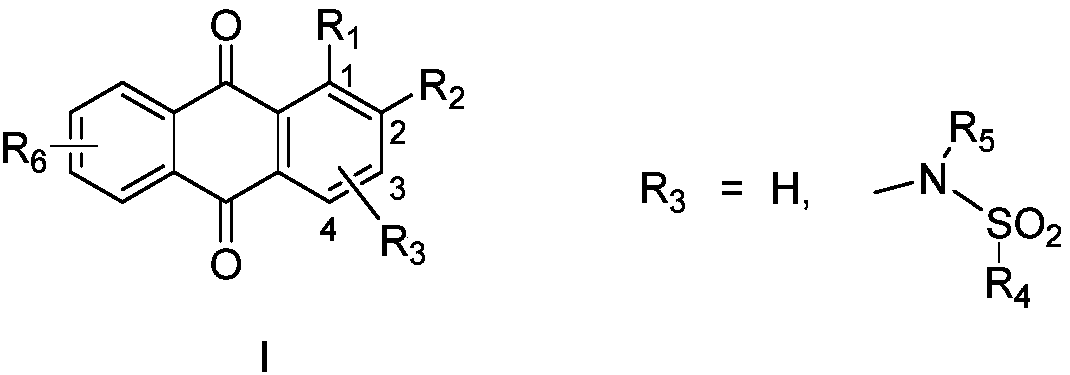
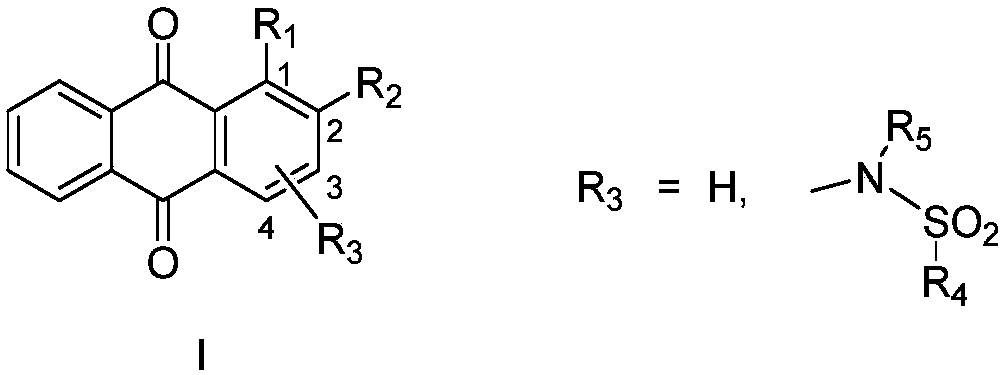9,10-anthraquinone compound, pharmaceutically-acceptable salts and pharmaceutical application thereof
A technology for anthraquinones and compounds, which is applied in the field of preparing cancer drugs, can solve problems such as failure to meet the standards of ready-made drugs, and achieve the effect of good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of diethyl 2,2'-((9,10-dioxo-9,10-dihydroanthracene-1,2-yl)dioxo)diacetate (1)
[0038]
[0039] To a 100 mL round bottom flask was added 1,2-dihydroxyanthracene-9,10-dione (1.2 g, 5 mmol), potassium carbonate (1.66 g, 12 mmol), ethyl bromoacetate (2 g, 12 mmol) and N,N -Dimethylformamide (50 mL), react at 80° C., TLC detects that the starting point disappears and stops the reaction. After the reaction solution was cooled to room temperature, the reaction solution was added dropwise to about 350 mL of 10% hydrochloric acid, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, and separated by silica gel column chromatography (petroleum ether: ethyl acetate =2:1), 0.89 g of yellow solid was obtained, and the yield was 43%. MS(ESI)(m / z):413.1(M-H) - . 1 HNMR (400MHz, DMSO-d 6 )δ8.13–8.07(m,2H),7.98(d,J=8.7Hz,1H),7.90–7.81(m,2H),7.50(d,J=8.8Hz,1H),5.04(s,2H ), 4.71 (s, 2H), 4.17 (qd, J=4.2, 7.2Hz, 4H), 1.20 (td...
Embodiment 2
[0041] Preparation of 2,2'-((9,10-dioxo-9,10-dihydroanthracene-1,2-yl)dioxo)diacetic acid (2)
[0042]
[0043] Add 1 (206mg, 0.5mmol), 0.5M lithium hydroxide aqueous solution (8mL) and methanol (8mL) into a 50mL round bottom flask, react at 30°C, TLC detects that the raw material point disappears to stop the reaction. Methanol was removed under reduced pressure, and the reaction solution was added dropwise to about 10% hydrochloric acid to adjust the pH to 1-2, and 169 mg of a yellow solid was precipitated, with a yield of 95%. MS(ESI)(m / z):355.0(M-H) - . 1 HNMR (400MHz, DMSO-d 6 )δ12.71(brs,2H),8.09(dt,J=1.4,7.3Hz,2H),7.96(d,J=8.7Hz,1H),7.84(m,2H),7.46(d,J=8.8 Hz, 1H), 4.93(s, 2H), 4.62(s, 2H).
Embodiment 3
[0045] Preparation of 2-((1-hydroxy-9,10-dioxo-9,10-dihydroanthracen-2-yl)oxy)-N,N-dimethylacetamide (3)
[0046] 1. Preparation of ethyl 2-((1-hydroxy-9,10-dioxo-9,10-dihydroanthracene-1,2-yl)oxy)acetate (HKb-2)
[0047]
[0048] The preparation method of HKb-2 was the same as that of 1, except that the amount of potassium carbonate was changed to 0.83 g, and the amount of ethyl bromoacetate was changed to 1 g to obtain an orange solid with a yield of 48%.
[0049] 2. Preparation of 2,-((1-hydroxy-9,10-dioxo-9,10-dihydroanthracene-1,2-yl)oxy)acetic acid (HKb-3)
[0050]
[0051] The preparation method of HKb-3 was the same as that of 2 to obtain an orange solid with a yield of 95%.
[0052] 3. Preparation of 2-((1-hydroxy-9,10-dioxo-9,10-dihydroanthracene-2-yl)oxy)-N,N-dimethylacetamide (3)
[0053]
[0054] Into a 50 mL round bottom flask was added HKb-3 (149 mg, 0.5 mmol), dimethylaminohydrochloride (31 mg, 0.68 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com