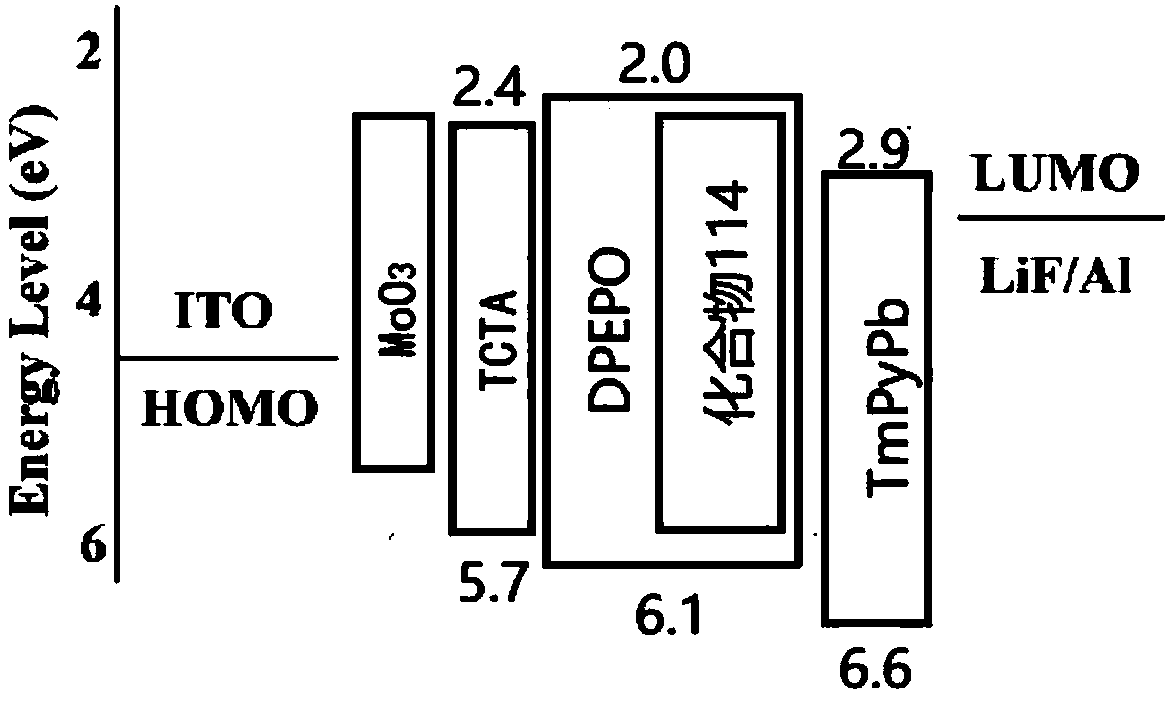
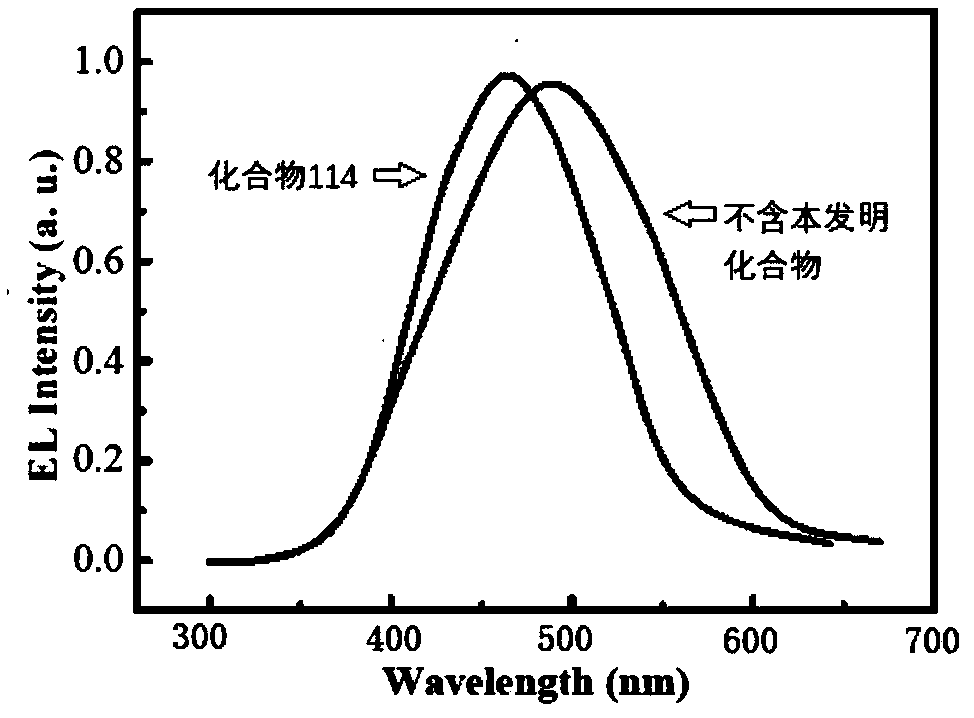
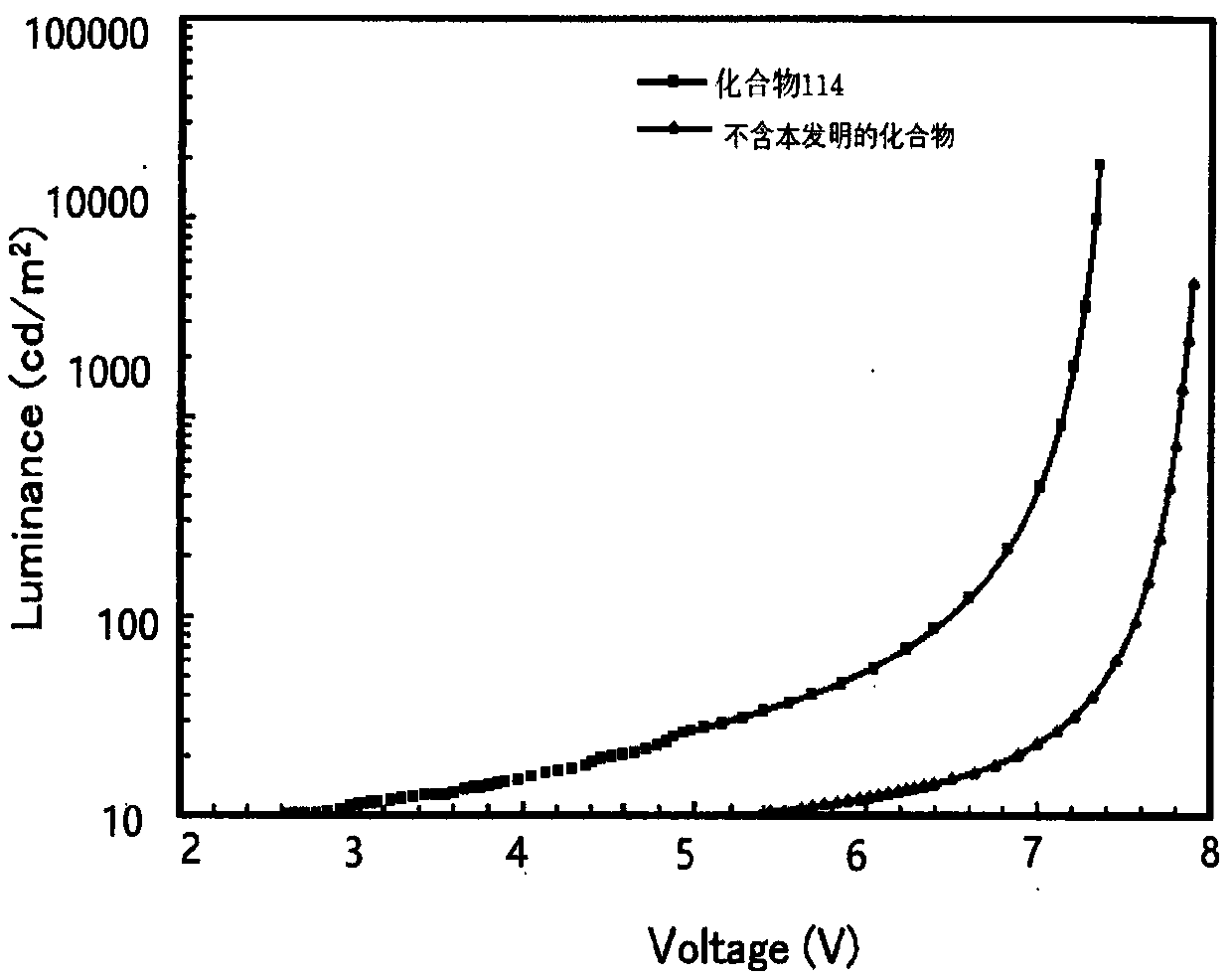
Organic optoelectronic material and application thereof
A technology of organic optoelectronic materials and light-emitting layers, applied in the fields of light-emitting materials, organic chemistry, circuits, etc., to achieve the effects of reducing the singlet-triplet energy gap, reducing overlap, and high exciton utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Compound 3 provided by the present invention can be synthesized by the following method.
[0049]
[0050] (1) In a 500ml three-necked flask, add 4,6-dichloro-1,3,5-triazin-2-amine (16.50g, 100mmol), 4-tert-butylphenylboronic acid (35.60g, 200mmol), Potassium carbonate (27.64g, 200mmol), toluene 150mL, ethanol 75mL, water 75mL, in N 2 Tetrakis(triphenylphosphine)palladium (0.35 g, 3 mmol) was added under protection, the reaction was controlled at 85° C., and the reaction was carried out for 12 h, and the reaction was completed by liquid phase monitoring. Cool to room temperature, wash twice with water, add activated carbon for decolorization, filter, concentrate to obtain a light yellow solid, recrystallize twice from ethanol, and dry under vacuum to obtain 4,6-bis(4-tert-butylphenyl)-1,3, 32.44 g of 5-triazin-2-amine, yield 90%.
[0051] (2) In a 500ml three-necked flask, add phenanthrenequinone (10.41g, 50mmol), 4,6-di(4-tert-butylphenyl)-1,3,5-triazin-2-amine (2...
Embodiment 2
[0053] Compound 12 provided by the present invention can be synthesized by the following method.
[0054]
[0055] (1) In a 500ml three-necked flask, add 4,6-dichloro-1,3,5-triazin-2-amine (16.50g, 100mmol), (9,9-dimethyl-9H-fluorene-2 -base) boric acid (47.62g, 200mmol), potassium carbonate (27.64g, 200mmol), toluene 150mL, ethanol 75mL, water 75mL, in N 2 Tetrakis(triphenylphosphine)palladium (0.35 g, 3 mmol) was added under protection, the reaction was controlled at 85° C., and the reaction was carried out for 12 h, and the reaction was completed by liquid phase monitoring. Cool to room temperature, wash twice with water, add activated carbon for decolorization, filter, concentrate to obtain a light yellow solid, recrystallize twice from ethanol, and dry under vacuum to obtain 4,6-bis(9,9-methyl-9H-fluorene-2- base)-1,3,5-triazin-2-amine 42.29g, yield 88%.
[0056] (2) In a 500ml three-necked flask, add phenanthrenequinone (10.41g, 50mmol), 4,6-di(9,9-methyl-9H-fluoren...
Embodiment 3
[0058] Compound 21 provided by the present invention can be synthesized by the following method.
[0059]
[0060] (1) In a 500ml three-necked flask, add 4,6-dichloro-1,3,5-triazin-2-amine (16.50g, 100mmol), phenylboronic acid (24.39g, 200mmol), potassium carbonate (27.64g , 200mmol), toluene 150mL, ethanol 75mL, water 75mL, in N 2 Tetrakis(triphenylphosphine)palladium (0.35 g, 3 mmol) was added under protection, the reaction was controlled at 85° C., and the reaction was carried out for 12 h, and the reaction was completed by liquid phase monitoring. Cool to room temperature, wash twice with water, add activated carbon for decolorization, filter, concentrate to obtain a light yellow solid, recrystallize twice from ethanol, and dry under vacuum to obtain 4,6-diphenyl-1,3,5-triazine-2- Amine 22.35g, yield 90%.
[0061] (2) In a 500ml three-necked flask, add phenanthrenequinone (10.41g, 50mmol), 4,6-diphenyl-1,3,5-triazin-2-amine (14.89g, 60mmol), [1,1 '-Biphenyl]-4-formal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com