Identification method and application of insulin mass spectrum peptide graph
A technology for insulin and human insulin, which is applied in the field of establishing the peptide map of insulin enzymatic hydrolysis mass spectrometry, which can solve the problems of difficulty, animal-derived insulin, and other problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
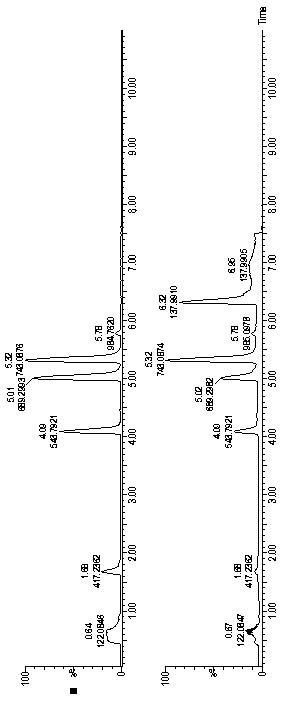
[0042] Mass spectrometry peptide map of recombinant human insulin, insulin aspart, insulin lispro and porcine insulin injection V8 enzymatic hydrolysis and mass spectrometry peptide map of V8 enzyme combined with lysyl endopeptidase
[0043] Take one injection sample each, transfer it to a 15ml centrifuge tube, adjust the pH value of the sample to 8.5 with a suitable Tris-Hcl buffer, and dilute the volume to 15ml.
[0044] (1) Take 600 μl of the diluted sample, add 200 μl of V8 enzyme (1 mg / ml), overnight at room temperature, and then add 400 μl of Tris-Hcl buffer;
[0045] (2) Take 600 μl of diluted sample, add 200 μl of V8 enzyme (1 mg / ml) and 50 μl of lysyl endopeptidase (20 μg / ml), leave at room temperature overnight, and then add 400 μl of Tris-Hcl buffer. Samples were diluted 20 times before injection. Using Waters ACQUITY UPLC ® CSH TM C18, 1.7μm, 50×2.1mm (I.D) chromatographic column, according to the gradient conditions in the following table, use 0.1% formic acid ...
Embodiment 2
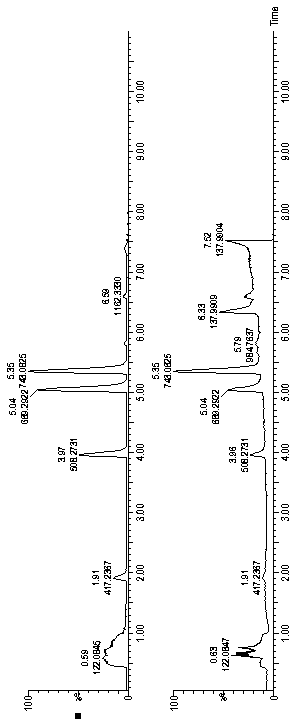
[0055] Determination of Recombinant Human Insulin and Insulin Lispro Injection V8 Enzyme and Lysyl Endopeptidase Enzyme Mass Spectrometry Peptide Mapping
[0056] Take one injection sample each, transfer it to a 15ml centrifuge tube, adjust the pH value of the sample to 9 with a suitable Tris-Hcl buffer, and dilute the volume to 15ml. Take 1000 μl of the diluted sample, add 100 μl of V8 enzyme (1 mg / ml) and 20 μl of lysyl endopeptidase (20 μg / ml), overnight at room temperature, and then add 400 μl of Tris-Hcl buffer. Samples were diluted 10 times before injection. Measured according to the gradient conditions of Example 1, using 0.7% formic acid aqueous solution as mobile phase A, acetonitrile as mobile phase B, 0.2ml / min flow rate, 55°C column temperature, 5 μl of sample injection, and collection of ion chromatogram; The four characteristic polypeptide fragments are shown in Table 7:
[0057] Table 7 Peptides hydrolyzed by combination of recombinant human insulin and insuli...
Embodiment 3
[0061] Determination of peptide map of insulin aspart injection and counterfeit insulin aspart injection V8 enzymatic hydrolysis mass spectrometry
[0062] Take one injection sample each, transfer it to a 15ml centrifuge tube, adjust the pH value of the sample to 7.5 with Tris-Hcl buffer, and dilute the volume to 15ml. Take 1000 μl of the diluted sample, add 200 μl of V8 enzyme (1 mg / ml), overnight at room temperature, and then add 400 μl of Tris-Hcl buffer. Samples were diluted 30 times before injection. According to the gradient conditions in Example 1, 0.5% formic acid aqueous solution was used as mobile phase A, acetonitrile was used as mobile phase B, the flow rate was 0.6 ml / min, the column temperature was 50°C, 5 μl was injected for measurement, and the ion chromatogram was collected. The four characteristic polypeptide fragments produced after enzymatic hydrolysis of the sample are shown in Table 8:
[0063] Table 8 Enzymatic hydrolysis peptides of insulin aspart and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com