Drug eluting balloon catheter and preparation method thereof
A technology of balloon catheters and drugs, which is applied in the direction of balloon catheters, catheters, drug delivery, etc., can solve the problems of strong adhesion between the hydrophobic coating and the surface of the balloon, and the difficulty of transferring drugs to tissues, so as to reduce the loss of drugs , Inhibit long-term restenosis and maintain integrity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
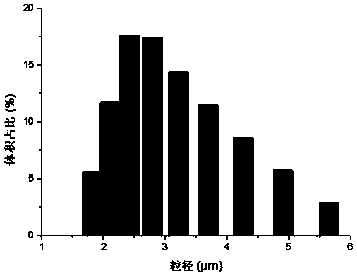
[0037] Weigh 10mg of rapamycin, dissolve it in 10ml of acetone, add this solution dropwise to 50ml of PVA solution with a mass fraction of 1%, and use ultrasonic emulsification at 4°C for 40 minutes, then stir the emulsion overnight at a speed of 1000rpm. After overnight, the emulsion was centrifuged at 3500rpm, and the supernatant was taken to obtain a suspension of rapamycin particles. The size and distribution of solid particles were tested with a nanoparticle size analyzer (Zetasizer Nano ZS90). The particle size range was 1.5 μm —6.0μm, the result is as follows image 3 .
Embodiment 2
[0039] Weigh 1 mg of rapamycin, dissolve it in 10 ml of acetone, add this solution dropwise to 100 ml of polysorbate 20 solution with a mass fraction of 2%, use ultrasonic emulsification at 4°C for 40 minutes, and then stir the emulsion overnight at Centrifuge the overnight emulsion at 3500rpm and take the supernatant to obtain a suspension of rapamycin particles. Use a nanoparticle size analyzer to test the size and distribution of solid particles. The particle size range is 0.5μm-5μm .
Embodiment 3
[0041] Weigh 100mg of rapamycin and 150mg of PDLGA with an intrinsic viscosity of 0.8dl / g, dissolve in 10ml of acetone and dichloromethane mixed solvent (volume ratio 2:8), add this solution dropwise to 100ml with a mass fraction of 2% polysorbate 80 solution, using a high-speed homogenizer to emulsify at 18000rpm for 4 minutes, then stir the emulsion overnight at 1000rpm, centrifuge the overnight emulsion at 3500rpm, take the supernatant, Both obtain the suspension of rapamycin particle, adopt the size and the distribution of solid particle test of nano particle size analyzer, particle size range 15nm-120nm, the result is as follows Figure 4 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com