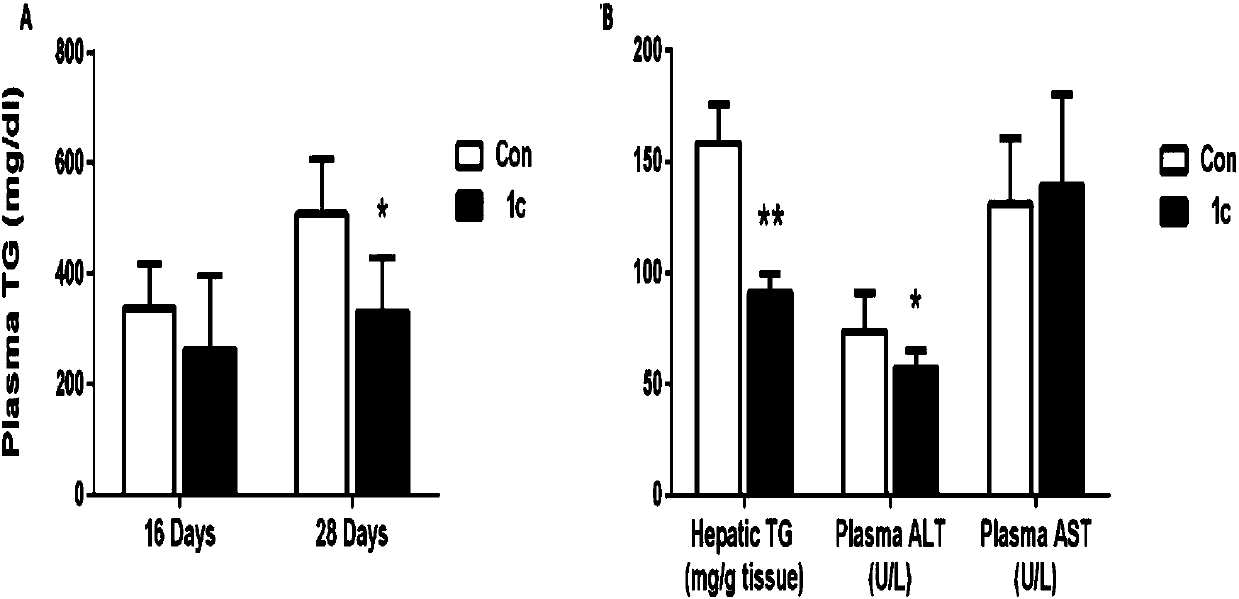
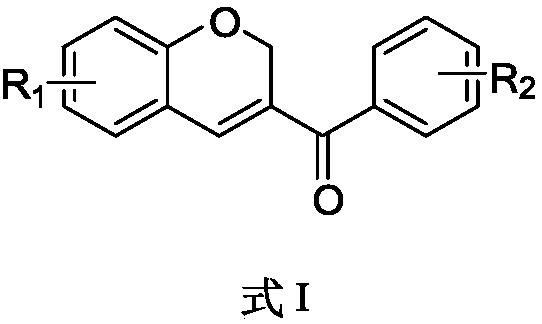
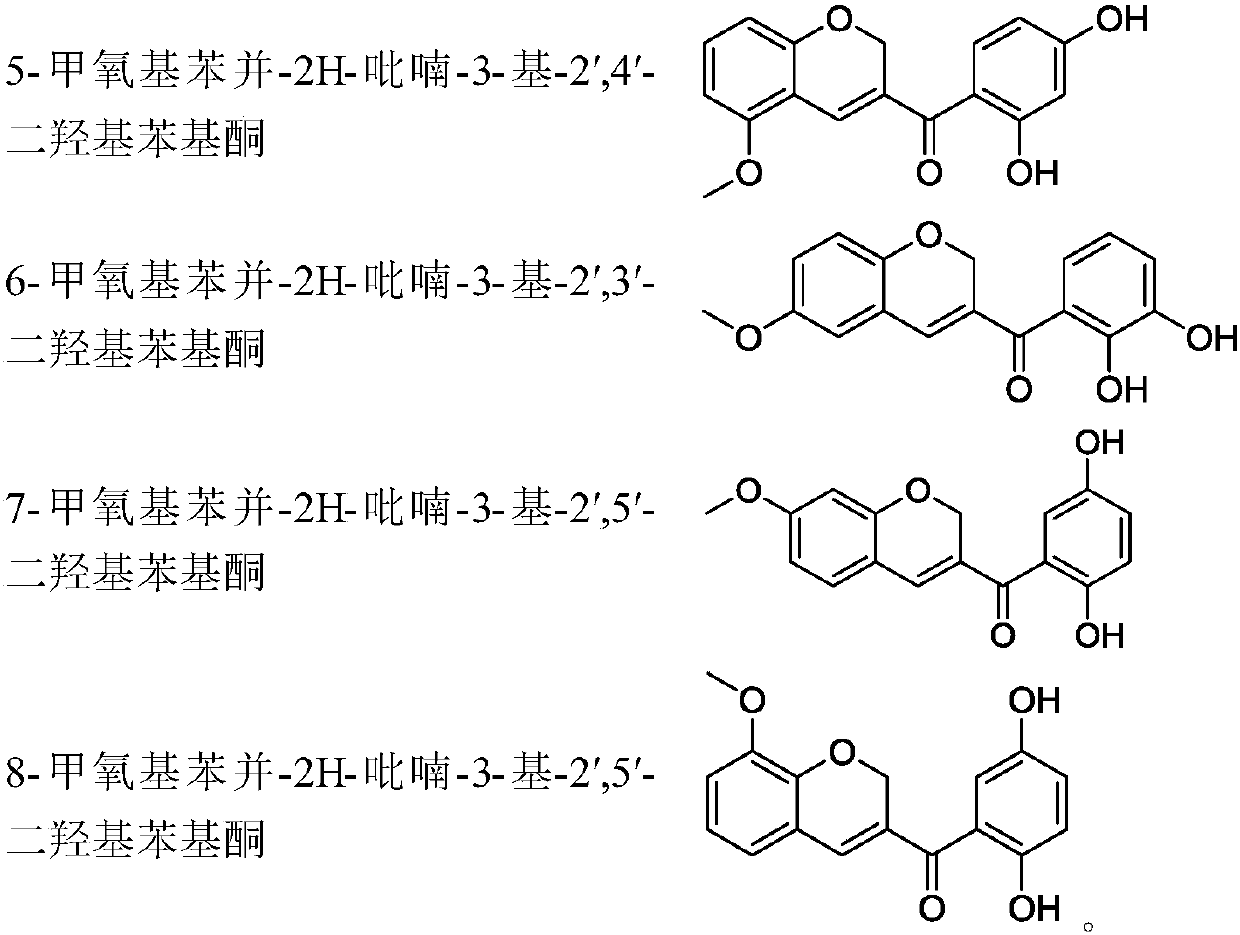
Benzopyran compounds and preparation method thereof as well as pharmaceutical composition and use of benzopyran compounds
A technology of benzopyran and compounds, applied in the field of benzopyran compounds, can solve problems such as lack, lack of triglyceride regulation, hypoglycemia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 6-methoxybenzo-2H-pyran-3-yl-2′,3′-dihydroxyphenyl ketone 1c
[0022]
[0023] (1) The preparation method of 6-methoxybenzopyran-3-carbonitrile
[0024] Dissolve 1g of 2-hydroxy-5-methoxybenzaldehyde in 2mL of acrylonitrile, add 2eq. of 1,4-diazabicyclo[2.2.2]octane, and reflux for 12h. The product 6-methoxybenzopyran-3-carbonitrile was obtained by column chromatography. 1 HNMR (500MHz, CDCl 3 ):7.13(s,1H),6.84(dd,J=9.0,3.0Hz,1H),6.82(d,J=9.0Hz,1H),6.63(d,J=2.5Hz,1H),4.75(s ,2H),3.77(s,1H); 13 CNMR (125MHz, CDCl 3 ):154.9,148.5,139.1,120.8,118.8,117.6,116.7,112.7,104.4,64.6,56.0; ESI-MS:[M+H] + :188.1,[M+NH 4 ] + :205.1.
[0025] (2) The preparation method of 6-methoxybenzo-2H-pyran-3-aldehyde
[0026] Under argon protection, 6-methoxychromene-3-carbonitrile was dissolved in toluene (150mg / mL), cooled to -50°C, added diisopropylaluminum hydride (1.1eq.) and stirred for 2h. Stirring was then continued for 2 h at room temperature. Add 100 mL of eth...
Embodiment 2
[0033] Example 2 5-methoxybenzo-2H-pyran-3-yl-2′,3′-dihydroxyphenyl ketone 1h
[0034]
[0035] (1) The preparation method of 5-methoxybenzopyran-3-carbonitrile
[0036] Dissolve 1g of 2-hydroxy-6-methoxybenzaldehyde in 2mL of acrylonitrile, add 2eq. of 1,4-diazabicyclo[2.2.2]octane, and reflux for 12h. The product 5-methoxybenzopyran-3-carbonitrile was obtained by column chromatography. 1 HNMR (500MHz, CDCl 3 ):7.54(s,1H),7.21(t,J=8.5Hz,1H),6.48(t,J=9.0Hz,2H),4.74(d,J=1.0Hz,2H),3.85(s,3H ); 13 CNMR (125MHz, CDCl 3 ):156.4, 155.2, 134.5, 133.0, 117.0, 110.3, 109.0, 104.1, 100.5, 63.9, 55.8; ESI-MS: [M+H] + :188.1,[M+NH 4 ] + :205.1.
[0037] (2) The preparation method of 5-methoxybenzo-2H-pyran-3-aldehyde
[0038] Under argon protection, 5-methoxychromene-3-carbonitrile was dissolved in toluene (150mg / mL), cooled to -50°C, added diisopropylaluminum hydride (1.1eq.) and stirred for 2h. Stirring was then continued for 2 h at room temperature. Add 100 mL of ethyl ac...
Embodiment 3
[0045] Example 3 8-methoxybenzo-2H-pyran-3-yl-2′,5′-dihydroxyphenyl ketone 1i
[0046]
[0047] (1) Preparation of 8-methoxybenzo-2H-pyran-3-carbonitrile
[0048] Dissolve 1g of 2-hydroxy-3-methoxybenzaldehyde in 2mL of acrylonitrile, add 2eq. of 1,4-diazabicyclo[2.2.2]octane, and reflux for 12h. The product 8-methoxybenzo-2H-pyran-3-carbonitrile was obtained by column chromatography. 1 HNMR (500MHz, CDCl 3 ):7.17(s,1H),6.93-6.91(m,2H),6.75-6.73(m,1H),4.87(d,J=1.0Hz,2H),3.88(s,1H); 13 CNMR (125MHz, CDCl 3 ):148.2, 143.4, 139.0, 122.3, 120.8, 120.4, 116.4, 115.4, 103.6, 64.7, 56.3; ESI-MS: [M+H] + :188.1,[M+NH 4 ] + :205.1.
[0049] (2) Preparation of 8-methoxybenzo-2H-pyran-3-yl-2′,5′-dihydroxyphenylketone
[0050] Add 8-methoxybenzo-2H-pyran-3-carbonitrile (0.2eq.) to the newly prepared Grignard reagent 2,5-bisMOMO-phenylmagnesium bromide, and reflux for 22h until the raw material disappears. Add 2N hydrochloric acid (2 mL) and reflux for 30 min. Add 100 mL of et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com