A kind of diarylethene photochromic conjugated polymer and preparation method thereof
A technology of diarylethene and conjugated polymers, which is applied in the field of polymers, can solve the problems of slow photochromic rate, etc., and achieve the effects of fast photochromic rate, good anti-fatigue performance, and sensitive photoresponse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0023] The preparation method of the diarylethene photochromic conjugated polymer in this embodiment is as follows:
[0024] Take 0.1mmol 1,2-bis(5-formyl-2-methylthiophen-3-yl)perfluorocyclopentene and 0.05mmol tetrakis(p-aminophenyl)methane, add to 0.75mL o-dichlorobenzene and In 2.25mL of n-butanol solution, ultrasonically disperse evenly for ten minutes, add 0.3mL of 3M acetic acid, react in a high-temperature reactor at 120°C for 5 days, wash and dry after natural cooling, and collect the photochromic polymer.
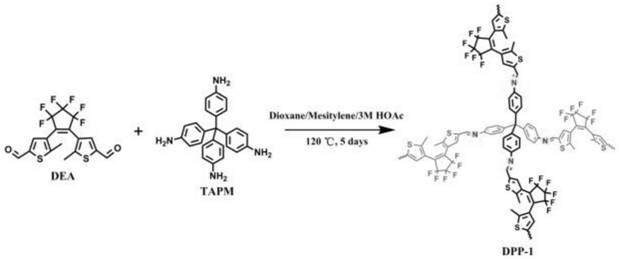
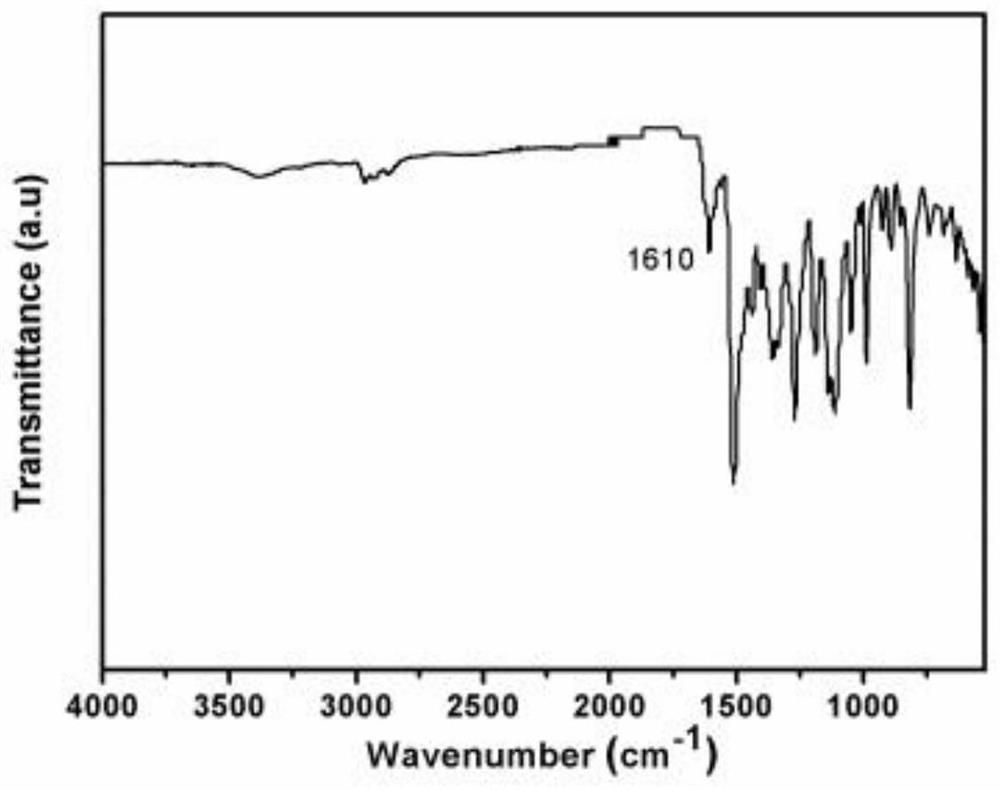
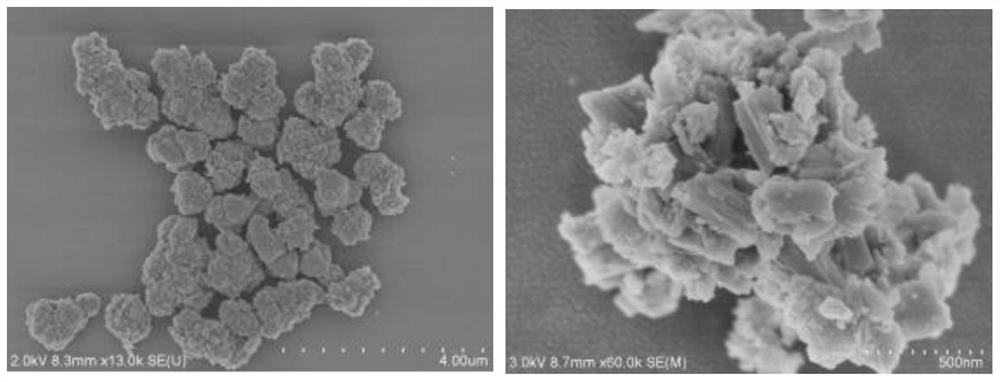
[0025] figure 1 It is the synthetic structure diagram of the diarylethene photochromic conjugated polymer obtained in step 1 of this embodiment, figure 2 is the infrared spectrum of the diarylethene photochromic conjugated polymer, image 3 A scanning electron microscope image.
[0026] Solid UV and cycle experiments
[0027] After being irradiated with 365nm ultraviolet light for different times, the ultraviolet spectrum of the diarylethene photochromic conj...
Embodiment 2
[0029] The preparation method of the diarylethene photochromic conjugated polymer in this embodiment is as follows:
[0030] Take 0.1mmol 1,2-bis(5-formyl-2-methylthiophen-3-yl) perfluorocyclopentene and 0.05mmol 4,4',4", 4"'-(pyrene-1,3 ,6,8-Tetrayl)tetraphenylamine, added to 0.75mL o-dichlorobenzene and 2.25mL ethanol solution, dispersed evenly by ultrasonication for ten minutes, then added 0.05g p-toluenesulfonic acid, and reacted in a high-temperature reaction kettle at 150°C After 3 days, after natural cooling, washing and drying, the photochromic polymer was collected.
[0031] Solid UV and cycle experiments
[0032] After being irradiated with 365nm ultraviolet light for different times, the ultraviolet spectrum of the diarylethene photochromic conjugated polymer at 200nm-900nm is measured. The cycle experiment is to measure the absorbance value of the polymer at 650 nm under the alternating irradiation of ultraviolet light and visible light. The ultraviolet spectrog...
Embodiment 3
[0034] The preparation method of the diarylethene photochromic conjugated polymer in this embodiment is as follows:
[0035]Take 0.1mmol 1,2-bis(5-formyl-2-methylthiophen-3-yl)perfluorocyclopentene and 0.05mmol 4,4',4",4"'-(ethylene-1,1 ,2,2-tetrayl)tetramine, added to 1.5mL of m-trimethylbenzene and 1.5mL of 1,4-dioxane solution, dispersed evenly by ultrasonication for 10 minutes, then added 0.3mL of 6M acetic acid, and reacted at 150°C React in the kettle for 5 days, wash and dry after natural cooling, and collect the photochromic polymer.
[0036] Solid UV and cycle experiments
[0037] After being irradiated with 365nm ultraviolet light for different times, the ultraviolet spectrum of the diarylethene photochromic conjugated polymer at 200nm-900nm is measured. The cycle experiment is to measure the absorbance value of the polymer at 650 nm under the alternating irradiation of ultraviolet light and visible light. The ultraviolet spectrogram shows that the color of the po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com