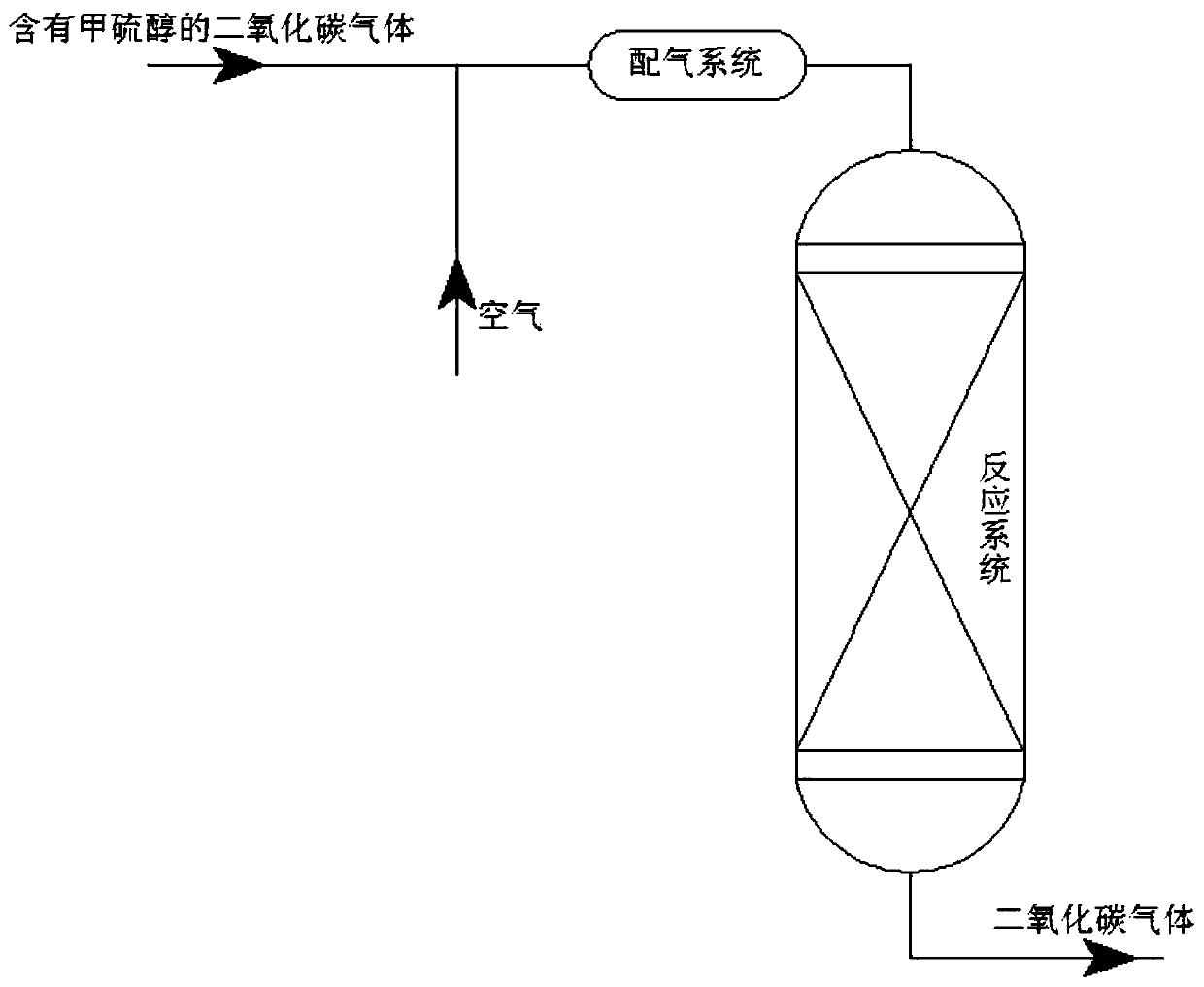
Process for removing methyl mercaptan from carbon dioxide gas
A carbon dioxide, methyl mercaptan technology, used in gas fuel, gas treatment, petroleum industry and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment includes the following steps:
[0030] Step 1, the carbon dioxide gas that the volume content of methyl mercaptan is 500ppm is passed in gas distribution system and mixes with air, obtains mixed gas; The mol ratio of oxygen and methyl mercaptan in described mixed gas is 1.8:1;
[0031] Step 2, feed the mixed gas obtained in step 1 into the reaction system, and carry out the methyl mercaptan removal reaction in the presence of a methyl mercaptan catalyst to obtain carbon dioxide gas; the pressure of the methyl mercaptan removal reaction is 4.0MPa , the temperature is 300℃, the space velocity of the mixed gas is 1000h -1 The demethylation mercaptan catalyst is prepared from waste zinc oxide desulfurizer, zinc nitrate, potassium permanganate, potassium hydroxide and activated clay, and the waste zinc oxide desulfurizer is zinc oxide desulfurizer after desulfurization reaction , the preparation process of the demethylation mercaptan catalyst is as follows: ...
Embodiment 2
[0033] This embodiment includes the following steps:
[0034] Step 1, the carbon dioxide gas that the volume content of methyl mercaptan is 1000ppm is passed in gas distribution system and mixes with air, obtains mixed gas; The mol ratio of oxygen and methyl mercaptan in described mixed gas is 0.8:1;
[0035] Step 2, feed the mixed gas obtained in step 1 into the reaction system, carry out the methyl mercaptan removal reaction under the condition that the methyl mercaptan removal catalyst exists, and obtain carbon dioxide gas; the pressure of the methyl mercaptan removal reaction is 3.0MPa , the temperature is 300℃, the space velocity of the mixed gas is 800h -1 The demethylation mercaptan catalyst is prepared from waste zinc oxide desulfurizer, basic zinc carbonate, manganese nitrate, potassium hydroxide and attapulgite, and the waste zinc oxide desulfurizer is zinc oxide desulfurization after desulfurization reaction agent, the preparation process of the demethylation merca...
Embodiment 3
[0037] This embodiment includes the following steps:
[0038] Step 1, the carbon dioxide gas that the volume content of methyl mercaptan is 100ppm is passed into gas distribution system and mixes with air, obtains mixed gas; The mol ratio of oxygen and methyl mercaptan in described mixed gas is 0.6:1;
[0039] Step 2, feed the mixed gas obtained in step 1 into the reaction system, and carry out the demethylation mercaptan reaction in the presence of a demethylation mercaptan catalyst to obtain carbon dioxide gas; the pressure of the demethylation mercaptan reaction is 0.1MPa , the temperature is 150℃, the space velocity of the mixed gas is 500h -1 The demethylation mercaptan catalyst is prepared from waste zinc oxide desulfurizer, zinc nitrate, potassium permanganate, potassium nitrate and bentonite, and the waste zinc oxide desulfurizer is zinc oxide desulfurizer after desulfurization reaction, so The preparation process of the demethylation mercaptan catalyst is as follows:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com